COVID-19 Vaccine Trial Data Shows a Negative Benefit to Harm Ratio in New Peer-Reviewed Paper
An important new paper was published in volume 40, issue 35 of the peer-reviewed Vaccine journal, with an impact score of 4.1, and an acceptance rate of 32%. (https://www.sciencedirect.com/science/article/pii/S0264410X22010283) (01)

One of the authors of the paper is Dr. Peter Doshi, an associate editor of the British Medical Journal. When he put his name to a pre-print of the paper, journalist Phil Harper reported (https://philharper.substack.com/p/peer-reviewed-vaccine-trial-data) it would never find its way through peer review, and yet here we are. (02)
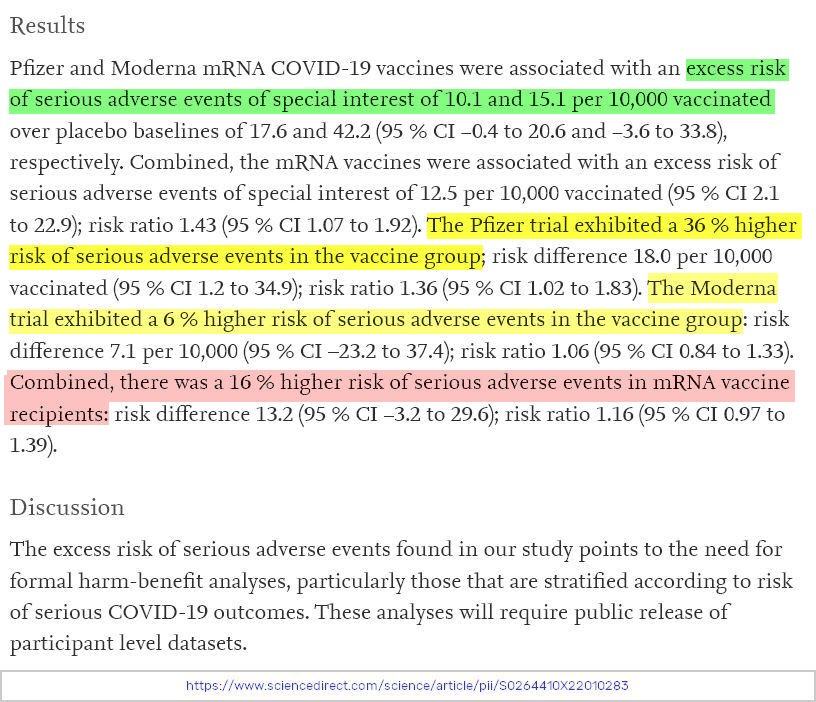
Put simply, both the Moderna and the Pfizer COVID-19 vaccine trial data appeared to have a negative benefit/harm ratio. Below is one of the key findings from the paper.
“In the Moderna trial, the excess risk of serious AESIs (15.1 per 10,000 participants) was higher than the risk reduction for COVID-19 hospitalization relative to the placebo group (6.4 per 10,000 participants).
In the Pfizer trial, the excess risk of serious AESIs (10.1 per 10,000) was higher than the risk reduction for COVID-19 hospitalization relative to the placebo group (2.3 per 10,000 participants).”
Quick jargon translation: AESI = adverse event of special interest. Or, in other words: serious adverse events which we should be paying very close attention to.
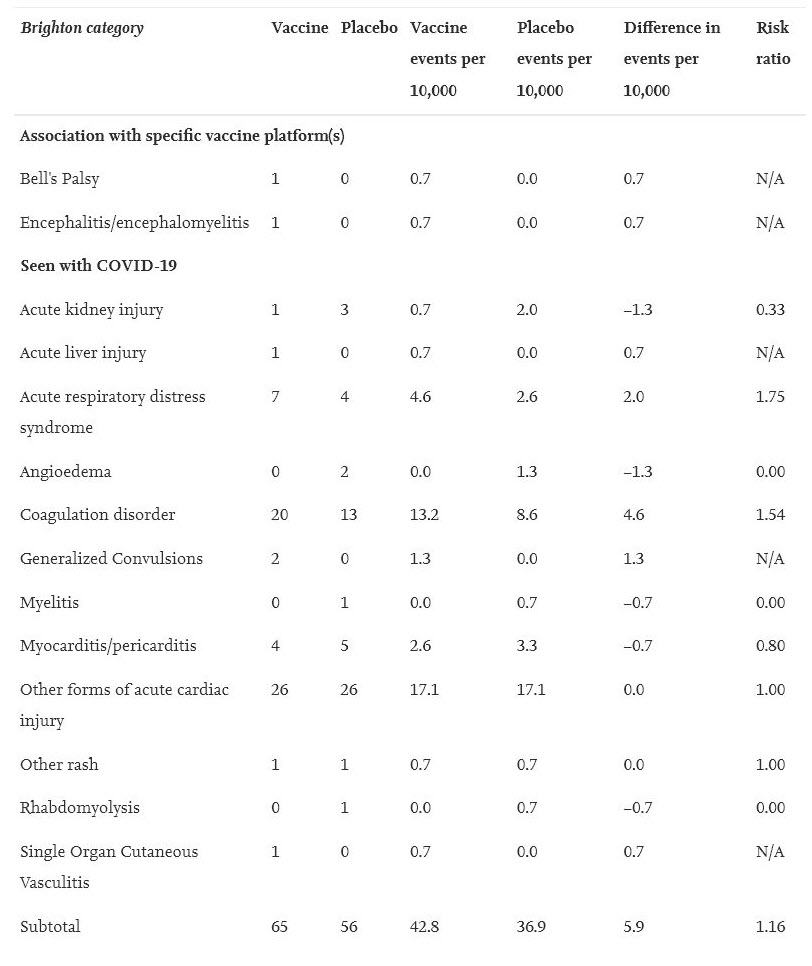
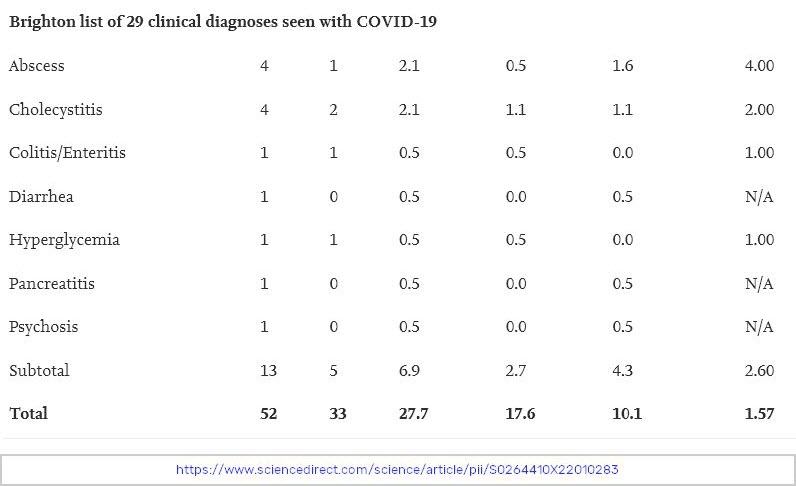
Serious Adverse Events are defined as “death; life-threatening at the time of the event; inpatient hospitalization or prolongation of existing hospitalization; persistent or significant disability/incapacity; a congenital anomaly/birth defect; medically important event, based on medical judgment.”
To find this data, investigators used four months of trial data submitted to regulators before the COVID-19 vaccines were put on the market. They gathered the data from journals, and data published by both Health Canada and the FDA. They created a blinded table of serious adverse events recorded in each trial and then had blinded investigators go through that table and determine if those serious adverse events were actually “AESIs”, defined above.
It’s important to be clear about what the findings of the paper suggest; trial data showed that mRNA vaccines appear to have a negative benefit/harm ratio.
Other key findings from the paper:
👉 There was a 16% higher risk of serious adverse events in mRNA vaccine recipients than in placebo recipients.
👉 There was a 43% higher risk of serious adverse events of special interest in mRNA vaccine recipients.
👉 mRNA vaccines are associated with more harm than initially estimated at the time of emergency authorization.
👉 Authors call for a formal risk-benefit analysis of the mRNA vaccines to take place.
👉 The authors suggest that we’ll need trial participant data in order to complete this task
👉Authors encourage third-party replication of their study, with access to complete SAE case narratives.
👉 The risks may be substantially less in some groups compared to others. Thus, knowing the actual demographics of those who experienced an increase in serious AESI in the vaccine group is necessary for a proper harm-benefit analysis.
For how much longer can the “safe and effective” mantra continue to exist?
Publication: Web | Download PDF | Supplementary Data (Word)
What to do if you’ve taken the jab?
- Clearing Spike Protein by Fasting (it’s the Only Way)
- 1 minute Spike-Protein Antidote (Dandelion)
- Time-Saving & Cheap Health Shortcuts
- If you are Forced to Get the Jab…
Posts tagged: Doctors | Myocarditis | Vaccines | Against our Will
Categories: Jab Victims | Rigged Science & Medicine | Pfizer


Site Notifications/Chat:
- Telegram Post Updates @JourneyToABetterLife (channel)
- Telegram Chatroom @JourneyBetterLifeCHAT (say hi / share info)
- Gettr Post Updates @chesaus (like fakebook)
Videos:
References















![[Police Standing Up] Steve – Former VIC Police](https://pennybutler.com/wp-content/uploads/2022/07/former-vic-police-steve-777x413.jpg)

