Paxlovid / Retonovir
- Updated:2 years ago
- Reading Time:23Minutes
- Post Words:6308Words
Scratchpad on Pfizer’s “COVID” Pill(s): Paxlovid / Retonovir – what a joke.
Living Document. First Published June 8, 2022 | Last updated: Feb 26, 2023
Paxlovid / Retonovir Videos:
2023:
Paxlovid ad on American TV
Interacts with medicines may cause life threatening side effects. Death?
Side effects may include altered taste, diarrhoea, high blood pressure, muscle aches, abdominal pain, nausea, feeling unwell, may affect how your birth control works, serious side effects can include hives, trouble swallowing or breathing, yellowing of the skin and eyes or dark urine. But please. Don’t risk Covid. Sponsored by Pfizer.
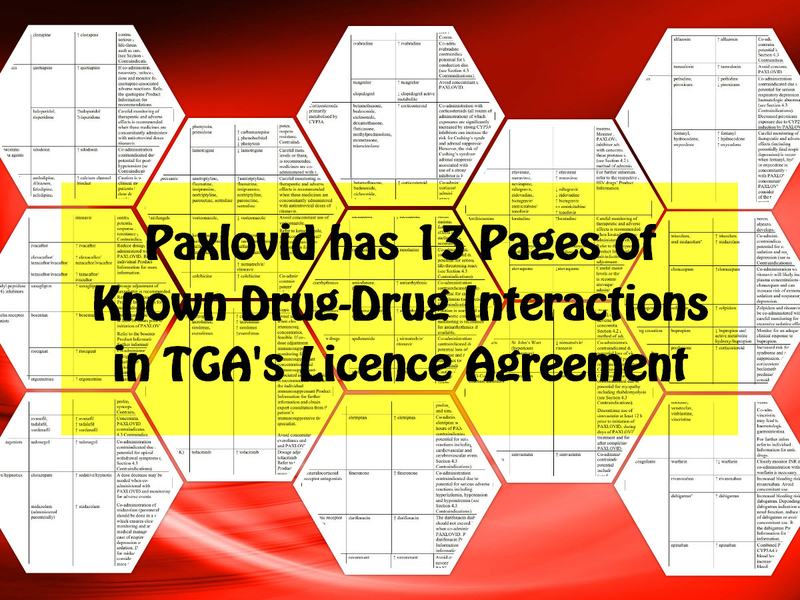
They seem to be missing a few from that ad, like the 13 pages of known drug-drug interactions in more than 20 different classes.
2022:
Paxlovid, is this an evidence based intervention in May 2022?
Dr John Campbell, 4 May 2022 | YouTube
Paper FDA EMU was based on:
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A, Pypstra R, Rusnak JM; EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022 Apr 14;386(15):1397-1408. doi: 10.1056/NEJMoa2118542. Epub 2022 Feb 16. PMID: 35172054; PMCID: PMC8908851. https://pubmed.ncbi.nlm.nih.gov/35172054/
- EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients)
- 1120 patients received nirmatrelvir plus ritonavir
- 1126 received placebo
- Relative risk reduction
- Risk of progression to severe Covid-19, 89% lower than the risk with placebo
- Absolute risk reduction
What is Paxlovid’s Absolute and Relative COVID-19 Risk Reduction
https://www.precisionvaccinations.com/what-paxlovids-absolute-and-relative-covid-19-risk-reduction
- 7% down to 1%
- symptomatic, unvaccinated, non hospitalized adults
- at high risk for progression to severe coronavirus disease 2019
- July 16 and December 9, 2021
- If vaccinated people and previously infected people are partly protected
- More people would need to be treated to prevent one adverse event
Pfizer press release (5th November 2021)
- our oral antiviral candidate, … has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations
Pfizer Shares In Vitro Efficacy of Novel COVID-19 Oral Treatment Against Omicron Variant
- https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-vitro-efficacy-novel-covid-19-oral-treatment
- https://www.fda.gov/media/155051/download
- 13 Things To Know About Paxlovid, the Latest COVID-19 Pill https://www.yalemedicine.org/news/13-things-to-know-paxlovid-covid-19
The results showed in all cases that nirmatrelvir was a potent inhibitor of its target.
PAXLOVID™ for Post-Exposure Prophylactic Use
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-top-line-results-phase-23-epic-pep-study
- Evaluated data from 2,957 adults
- Pfizer observed risk reductions of 32% (5 day course)
- 37% reduction (10 day course)
- These results, however, were not statistically significant and, as such, the primary endpoint of reducing the risk of confirmed and symptomatic COVID-19 infection in adults who had been exposed to the virus through a household contact was not met.
FDA FACT SHEET for patients, parents, and caregivers | Emergency Use Authorization (EUA) of Paxlovid for coronavirus disease 2019 (COVID-19)
https://www.fda.gov/media/155051/download
- PAXLOVID is not an FDA-approved medicine in the United States.
- PAXLOVID is an investigational medicine
- Some medicines may interact with PAXLOVID and may cause serious side effects.
- If you take too much PAXLOVID, call your healthcare provider or go to the nearest hospital emergency room right away.
Possible side effects of PAXLOVID are:
- Allergic Reactions trouble swallowing or breathing, swelling of the mouth, lips, or face, throat tightness, hoarseness, skin rash
- Liver Problems. Tell your healthcare provider right away, loss of appetite, yellowing of your skin and the whites of eyes (jaundice), dark-colored urine, pale colored stools and itchy skin, stomach area (abdominal) pain.
- Other possible side effects include: altered sense of taste, diarrhea, high blood pressure, muscle aches
- Dr. Ashish K. Jha, White House, Covid-19 coordinator
- Senior Advisor at Albright Stonebridge Group
- The new White House Covid czar says avoiding all virus infections isn’t the goal of U.S. pandemic policy.
- Paxlovid, push to reach the vulnerable
- Doctors are too hesitant to prescribe the drug
- The firm advises clients on international policy and global markets
- Patrick Vallance From 2012 to 2018, he was President of Research and Development at global pharmaceutical company, GlaxoSmithKline (GSK)
- Cashed £5,000,000 GSK shares
- Future jobs of FDA’s haematology-oncology reviewers BMJ 2016; 354 :i5055 doi:10.1136/bmj.i5055 | Bien J, Prasad V.
- When regulators leave government, some work or consult for the industries they regulated. Although this “revolving door” has been criticised, it has not been studied. We sought to measure how often it occurs at the FDA.
- A Look At How The Revolving Door Spins From FDA To Industry
- More than a quarter of the Food and Drug Administration employees (who approved cancer and hematology drugs from 2001 through 2010) left the agency and now work or consult for pharmaceutical companies.
Managing Paxlovid Drug Interactions
Liverpool Drug Interactions – Mar 3, 2022 YouTube
Paxlovid Failed to Prevent COVID-19
Dr Been May 11, 2022, YouTube | SubStack | Rumble
See also his Jan 5 2022 video “Paxlovid – How does it work?”
Paxlovid failed to provide prophylactic protection for the household members of the COVID patients. Let’s review the good, the bad, and the ugly of Paxlovid.
EPIC-HR: Study of Oral PF-07321332/Ritonavir Compared With Placebo in Nonhospitalized High Risk Adults With COVID-19 – Full Text View – ClinicalTrials.gov
https://clinicaltrials.gov/ct2/show/NCT04960202
Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19 | NEJM
https://www.nejm.org/doi/full/10.1056/NEJMoa2118542
Clinical Studies | PAXLOVID™ (nirmatrelvir tablets; ritonavir tablets)
https://www.covid19oralrx-hcp.com/clinical-studies?source=google&HBX_PK=s_paxlovid+results&skwid=43700068281649038&gclid=Cj0KCQjwmuiTBhDoARIsAPiv6L_CQ8C5C7AQsXGYtMhZx5rjk7W8pNjd3gdK_Z1BYWY8X7Ifv_-iu5UaAm6-EALw_wcB&gclsrc=aw.ds
Paxlovid HCP FS 04142022
https://www.fda.gov/media/155050/download
EUA 105 Pfizer Paxlovid DHCP 04142022
https://www.fda.gov/media/155071/download
Pfizer Shares Top-Line Results from Phase 2/3 EPIC-PEP Study of PAXLOVID™ for Post-Exposure Prophylactic Use | Pfizer
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-top-line-results-phase-23-epic-pep-study
Paxlovid Mouth Is Real—And Gross – The Atlantic
https://www.theatlantic.com/health/archive/2022/05/pfizer-paxlovid-covid-pill-side-effects/629772/
Pfizer says COVID treatment Paxlovid fails to prevent infection of household members | Reuters
https://www.reuters.com/business/healthcare-pharmaceuticals/pfizer-says-covid-treatment-paxlovid-fails-prevent-infection-household-members-2022-04-29/
FDA warns Paxlovid as COVID treatment option
https://www.fda.gov/media/155194/download
Covid-19 cases that return after Paxlovid antiviral treatment puzzle doctors – CNN
https://edition.cnn.com/2022/04/27/health/paxlovid-covid-rebound/index.html
FDA EUA Note for Paxlovid
https://www.fda.gov/media/155194/download
May 2022 | Pfizer COVID Pill added to PBS | Australia
- Commencing 1 May 2022 Anti-COVID Pill added to the PBS (Lagevrio (Molnupiravir), Paxlovid)
- “Australians will only pay $42.50 per script or $6.80 if using concession card”
Clinical criteria for PBS listed Lagevrio and Paxlovid:
- People 65 years or older with two additional high-risk factors for developing severe disease,
- People 75 years or older with one additional high-risk factor for developing severe disease,
- Moderately to severely immunocompromised people irrespective of vaccination status, and
- Aboriginal and Torres Strait Islander people aged 50 years or older with two additional high-risk factors for developing severe disease.
Priority communities
Initial distribution of the oral treatments by the Australian Government was prioritised to those with the highest clinical need including:
- people living in residential aged care facilities
- rural and remote communities
- Aboriginal and Torres Strait Islander people
- people with a disability, especially in a supported living setting.
Articles:
- The Vulnerable & Unvaccinated will be Prioritized – The Guardian – Feb 2022 https://www.theguardian.com/australia-news/2022/feb/10/covid-19-pills-vulnerable-and-unvaccinated-australians-to-be-prioritised-for-new-oral-treatments
Drug Info: (Australia)
- https://www.nps.org.au/coronavirus/antiviral-treatments-for-covid-19
- https://www.health.gov.au/health-alerts/covid-19/treatments/oral
PBS Listing: (Australia)
- https://www.pbs.gov.au/info/news/2022/04/paxlovid-nirmatrelvir-and-ritonavir-pbs-listing
- https://www.pbs.gov.au/info/news/2022/03/lagevrio-molnupiravir-pbs-listing
PBS Listing: (USA)
FDA Evaluating Reports of COVID Rebound Symptoms After Taking Paxlovid.
April 29, 2022 Rumble
Frontline Flash™ Daily Dose: ‘Ivermectin vs Paxlovid: No Contest’ with Dr. Peterson Pierre
May 24, 2022 Rumble
Frontline Flash™ by AFLDS.org (America’s Frontline Doctors) with Dr. Peterson Pierre presents Daily Dose: ‘Ivermectin vs Paxlovid: No Contest’ (Ep. 2086 – 5.24.2022). The Real Story of Good Health ~ in 120 Seconds or Less.
- Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects Using Propensity Score Matching. Cureus, 14(1), e21272. https://doi.org/10.7759/cureus.21272 Kerr, L., Cadegiani, F. A., Baldi, F., Lobo, R. B., Assagra, W., Proença, F. C., Kory, P., Hibberd, J. A., & Chamie-Quintero, J. J. (2022).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8765582/ - Pfizer Says Its COVID-19 Pill Paxlovid Fails to Prevent Symptomatic Infection of Household Members | April 30, 2022 } Epoch Times (Paywalled – See Archived Version) https://www.theepochtimes.com/pfizer-says-its-covid-19-pill-paxlovid-fails-to-prevent-symptomatic-infection-of-household-members_4437625.html
Pfizer’s Paxlovid Covid REBOUND Problem Is Confusing Scientists
Kim Iversen – May 17, 2022 – Rumble
Government Pushes Doctors/Pharmacist to Dispense PAXLOVID and Ignore Major Side Effects
Feb 17, 2022 | Rumble
Pharmacist’s experience with the government : The government is pushing doctors to prescribe Pfizers’ new Covid pill (PAXLOVID) and to have pharmacists dispense it while ignoring the drug interactions and adverse side effects. In this video I will explain why the government has botched the roll out of this experimental medication and why it never should have been authorized in the first place. Many will suffer from unknown drug interactions that were overlooked.
Is Pfizer’s new oral COVID treatment PAXLOVID worth the money?
Dr. Marcum May 6, 2022 Rumble
There’s a new oral antiviral treatment drug for COVID-19 from Pfizer, recently made available with an emergency use authorization… but is it any good? In this video, Dr. Marcum breaks down a key statistic in Pfizer’s study, along with the side effects of Paxlovid, and shares his opinion on the initial data.
U.S. COVID Response One Of The Greatest Frauds
The Still Report: May 12, 2022 | Rumble
Dr. Michael B. Goodkin, MD, FACC, just published a blistering indictment of the U.S. government response to COVID in TrialSite News.
This is the best and most succinct summary of this God-awful, convoluted miasma of lies, deception and autocratic censorship in the history of medicine – and it still has not come to an end.
The following is Dr. Goodkin’s analysis of the last 2+ years of COVID.
- “Relatively few Americans received any therapy for early COVID. NIH studied multiple drug company therapeutic products but sabotaged, ignored or delayed the use of all repurposed (existing) drugs for early COVID.
- “It was done because successful repurposed drugs would have caused vaccine hesitancy and negated any rationale for emergency use authorizations for vaccines with inadequate safety and efficacy data. NIH has a conflict of interest by owning part of the Moderna patent.
- The government continues to prevent the use of repurposed drugs so that drug companies can make obscene profits on their new therapeutic products. The mRNA vaccines have caused great harm which the government, Pfizer and Moderna with the aid of the media and social media have covered up.
- “The government mandated vaccines for as many as they could despite it being well known that almost half the country had had previous infection, had better immunity than those who were vaccinated, posed no risk to the vaccinated and had little if anything to gain from being vaccinated.
- “These actions resulted in massive amounts of unnecessary illness, death, economic damage and harm to the wellbeing of Americans from not receiving the effective repurposed drugs which were available and being injured or killed by the mRNA vaccines.
- “Despite evidence of markedly decreasing efficacy the government continues to push more and more vaccine doses. Now that drug company therapeutics are available, repurposed drugs are still being sabotaged so that drug companies can make obscene profits on their therapeutics.
The supporting data will show:
- ) Government healthcare agencies sabotaged hydroxychloroquine which should have been in wide use for early COVID by June 2020 and ivermectin which should have been in use by January 2021. These drugs would have had a profound benefit on the pandemic.
- The drugs have no significant toxicity and there was no appreciable risk to their use. The government healthcare agencies organized a propaganda campaign against HCQ and IVM involving the media and social media, got pharmacies not to sell them and weaponized medical review boards to punish doctors who ordered them. These are FDA approved drugs being used off label which is the case for 20% of all prescriptions.
- The government encouraged hospitals to fight in court to prevent families from getting ivermectin for their desperately ill loved ones. For the families who won in court, their loved one usually lived. For those who lost, their loved one almost always died.
- Meanwhile the government paid hospitals 20% extra on the entire hospital bill to treat patients with remdesivir which at best has no mortality benefit and has slight benefit in shortening hospital stay, it has toxicity and WHO recommends against using it. We also pay hospitals more when COVID patients die. Hospitals have not done well during the pandemic but surely there were better ways to subsidize them. There was no reason to subsidize Gilead.
- ) Government healthcare agencies ignored excellent data on generic fluvoxamine, known by 8/6/21 and published in Lance Global Health 10/27/21. It would have had a marked benefit on the delta variant, having shown benefit against the even worse gamma variant. The FDA never acted on an EUA filed 12/21/21.
- ) NIH failed to do anything with over-the-counter famotidine (P epcid) which blocks H2 receptors on mast cells and has value in preventing cytokine storms. It and other over the counter mast cell therapies could have had profound benefits all over the world. The American Academy of Allergy Asthma and Immunology was very interested in January 2021. After they contacted the coronavirus taskforce, they lost all interest. At their national meeting in February 2022, one would not know that COVID had anything to do with mast cells. Those initially involved in January 2021 were president Dr, Giselle Mosnaim and head of research Dr. Mariana Castells. Most likely there is knowledge of it by secretary-treasurer Dr. Jonathan Bernstein and head of mast cells Dr. Anne Maitland. They should all be questioned regarding government interference.
- ) Other promising therapies like generic spironolactone and branded antiandrogen proxalutamide, which lowered admissions 91% and shortened illness from 21.8 to 4.2 days, have been ignored.
- ) Several groups reported terrific results with combinations of drugs, but no drug combination was ever studied. No major medical institution did much of anything with combination therapy and went along with no treatment for early COVID. Some of it was related to them never considering the possibility of fraud by NIH, FDA and CDC. Some was related to drug company influence. Some appear to be related to fear of government reprisals and loss of grant money.
- ) The repurposed drugs have been pushed aside for monoclonal antibodies, molnupiravir and paxlovid. Molnupiravir’s efficacy ($700) was slightly worse than fluvoxamine ($10) and has the potential of causing genetic defects and causing worse variants but unlike fluvoxamine got an EUA. Paxlovid ($530) was 89% effective in the one and only study run by Pfizer in preventing hospitalization and got an EUA, but few are getting it. It is effective but hasn’t performed as well in practice. It has a bad metallic taste. Some patients get recurrent COVID after treatment. It interacts with many other drugs. It had no specific data on treatment against omicron and BA.2.
- ) mRNA vaccines appear to have major toxicity which has been suppressed. Our government spent $1 billion to advertise the vaccines in the media and social media and had them suppress important safety information.
- ) Three (3) military physicians reviewed the DMED data and found massive increases in many diagnoses, hypertension, pulmonary embolus, miscarriages and cancer among many others. The Department of Defense is trying to cover it up. Senator Ron Johnson is aware of what is going on as are other republicans.
- ) Insurance company data for the second half of 2021 shows a 40% increase in all-cause mortality in those 18-64 years. Mortality is up 84% in millennials. Data from England shows that in the vaccinated, all-cause mortality is increased in all age groups except those over 90 years old.
- The FDA restricted the use of the viral vector Johnson and Johnson vaccine due the rare development of TTP. The vaccine is effective in preventing COVID and decreases all-cause mortality. Novavax vaccine which is protein based has good efficacy data and decreases all-cause mortality but is being held up by the FDA.
- ) The Pfizer data that a court forced the FDA to finally release shows massive deception and out and out fraud.
CONCLUSIONS
- Our government has been involved in what I consider one of the greatest frauds in modern history. It has caused immense damage not just in the US but across the planet.
- I believe it is the job of the GAO to investigate, make your conclusions known to the public, help bring those responsible to account and help reform the system which allowed something like this to happen in the first place.
- A Letter To The GAO: Government Suppression of Early COVID-19 Treatment (PayWalled – Download PDF Mirror from Locals) https://www.trialsitenews.com/a/a-letter-to-the-gao-government-suppression-of-early-covid-19-treatment-5d21302f Michael B. Goodkin MD, FA
CDC Issues Warning on Pfizer’s COVID Pills | Facts Matter
EpochTV, June 3, 2022 | Rumble | TheEpochTimes
The CDC recently issued a warning regarding Pfizer’s COVID-19 pills—the ones that were approved last year and sold under the name Paxlovid.
People usually take those pills after they contract COVID-19 to lessen the symptoms and lower the chance of hospitalization.
However, a new advisory from the CDC warns Americans that taking Paxlovid can actually lead to a rebound in COVID-19 symptoms—which is a problem that, according to new documentation from the FDA, they were aware of even during the clinical trial phase.
Memory-Holed: The WHO Document from 2015 That Can No Longer Be Found
Dr Tess Lawrie: June 3, 2022 | Rumble | Full Video RedVoiceMedia (3.5hrs)
What is the significance of this Document?
- Facilitated early dissemination of big pharma’s “results” in the media.
- Facilitated publication of big pharma trials in favoured journals.
- Facilitated emergency use authorization of remdesivir, molnupiravir, paxlovid, and COVID-19 gene-based vaccines without independent peer review and adequate safety data.
- BUT for ivermectin, only negative studies were published, with hindrance of study publication and discrediting of scientists who reported positive results.
Dr. Tess Lawrie: “According to that document, ivermectin should be authorized, at the very least given emergency use authorization like the new drugs that are being offered, but the page has now been taken down.”
When Pfizer gets AUD$1114-$2215 per script for Paxlovid, it explains why so much was spent demonizing Ivermectin, which is off-patent and costs cents. The Covid Pandemic Skyrockets Pfizer’s 2022 Revenue To A Record $100 Billion: $37.8 billion made on Covid vaccines. $18.9 billion made on Paxlovid anti-viral pill. (01) CNBC The Covid pandemic drives Pfizer’s 2022 revenue to a record $100 billion https://www.cnbc.com/amp/2023/01/31/the-covid-pandemic-drives-pfizers-2022-revenue-to-a-record-100-billion.html (02) Australian Paxlovid PBS Pricing – NIRMATRELVIR (&) RITONAVIR – https://www.pbs.gov.au/medicine/item/12996B-13147Y
Paxlovid / Retonovir Links:
Download my Paxlovid folder on Mega
TGA’s Paxlovid Labelling Exemption 2022
Last updated: 27 January 2022
“I, John Skerritt, as the appropriate authority, grant the following labelling exemption. Dated 20 January 2022″ “This labelling exemption is granted under section 1.5.5 of Part 2 of the current Poisons Standard.” (03)Therapeutic Goods (Poisons Standard) (COVID-19 Treatment – Pfizer) (Nirmatrelvir and Ritonavir) Labelling Exemption 2022 … Click for full citation
Ritonavir-Boosted Nirmatrelvir (Paxlovid)
Last Updated: May 13, 2022
- The most common adverse effects of ritonavir-boosted nirmatrelvir are dysgeusia, diarrhea, hypertension, and myalgia.
- Ritonavir-boosted nirmatrelvir has significant drug-drug interactions, primarily due to the ritonavir component of the combination.
Drug-Drug Interactions Between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Concomitant Medications Last Updated: May 13, 2022 https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir–paxlovid-/paxlovid-drug-drug-interactions/
Too many to list here.
COVID-19 Rebound After Paxlovid Treatment
May 24, 2022
https://emergency.cdc.gov/han/2022/pdf/CDC_HAN_467.pdf
COVID-19 rebound has been reported to occur between 2 and 8 days after initial recovery and is characterized by a recurrence of COVID-19 symptoms or a new positive viral test after having tested negative.
FDA Fact Sheet for EUA for Paxlovid
Last Updated: 14 April 2022
https://www.fda.gov/media/155050/download
Warnings:
- The concomitant use of PAXLOVID and certain other drugs may result in potentially significant drug interactions. Consult the full prescribing information prior to and during treatment for potential drug interactions.
- Allergic Reactions/Hypersensitivity: Hypersensitivity reactions have been reported with PAXLOVID. If signs and symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, immediately discontinue PAXLOVID and initiate appropriate medications and/or supportive care.
- Hepatotoxicity: Hepatic transaminase elevations, clinical hepatitis, and jaundice have occurred in patients receiving ritonavir.
- HIV-1 Drug Resistance: PAXLOVID use may lead to a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection.
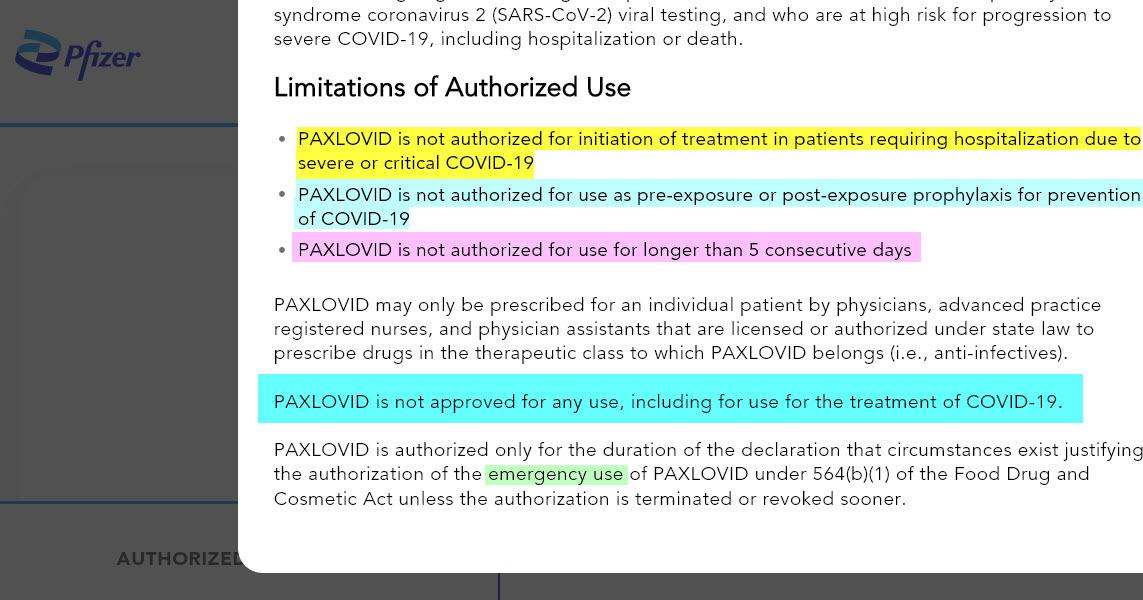
Limitations of Authorized Use:
- PAXLOVID is not authorized for initiation of treatment in patients requiring hospitalization due to severe or critical COVID-19.
- PAXLOVID is not authorized for use as pre-exposure or post-exposure prophylaxis for prevention of COVID-19.
- PAXLOVID is not authorized for use for longer than 5 consecutive days.
- PAXLOVID is not approved for any use, including for use for the treatment of COVID-19.
- PAXLOVID is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of PAXLOVID under section 564(b)(1) of the Act, 21 U.S.C.§ 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
- PAXLOVID is not recommended in patients with severe renal impairment
- There are limited clinical data available for PAXLOVID. Serious and unexpected adverse events may occur that have not been previously reported with PAXLOVID use.
- Hypersensitivity reactions have been reported with PAXLOVID including urticaria, angioedema, dyspnea, mild skin eruptions, and pruritus. Cases of anaphylaxis, TEN, and Stevens-Johnson syndrome have also been reported with ritonavir, a component of PAXLOVID. If signs and symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, immediately discontinue PAXLOVID and initiate appropriate medications and/or supportive care.
Hmm… Pox? Wow. Compare pruritus, TEN, and Stevens-Johnson syndrome to MonkeyPox.
- Hepatic transaminase elevations, clinical hepatitis, and jaundice have occurred in patients receiving ritonavir. Therefore, caution should be exercised when administering PAXLOVID to patients with pre-existing liver diseases, liver enzyme abnormalities, or hepatitis.
Risk of HIV-1 Resistance Development
- Because nirmatrelvir is co-administered with ritonavir, there may be a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection
6 pages of known Potentially Significant drug-interactions (Pages 9-15)
Systemic exposure of nirmatrelvir increases in renally impaired patients with increase in the severity of renal impairment.
PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets. Nirmatrelvir is a SARS-CoV-2 main protease (Mpro) inhibitor, and ritonavir is an HIV-1 protease inhibitor and CYP3A inhibitor.
The pharmacokinetics of nirmatrelvir/ritonavir in patients less than 18 years of age have not been evaluated.
Carcinogenicity studies have not been conducted with nirmatrelvir.
- Paxlovid HCP FS 04142022
https://www.fda.gov/media/155050/download - EUA 105 Pfizer Paxlovid DHCP 04142022
https://www.fda.gov/media/155071/download - FDA warns Paxlovid as COVID treatment option
https://www.fda.gov/media/155194/download - PAXLOVID Patient Eligibility Screening Checklist Tool for Prescribers
https://www.fda.gov/media/158165/download
Management of Drug Interactions With Nirmatrelvir/Ritonavir (Paxlovid®): Resource for Clinicians (last updated, May 6, 2022)
https://www.idsociety.org/globalassets/idsa/practice-guidelines/covid-19/treatment/idsa-paxlovid-drug-interactions-resource-5-6-22-v1.1.pdf
LABEL: PAXLOVID- nirmatrelvir and ritonavir kit
Packager: Pfizer Laboratories Div Pfizer Inc
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bdddfba-bd31-44cb-ba9e-23a4e17a4691
- There are no available human data on the use of nirmatrelvir during pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
- Systemic exposure of nirmatrelvir increases in renally impaired patients with increase in the severity of renal impairment
- Carcinogenicity studies have not been conducted with nirmatrelvir.
Nirmatrelvir/ritonavir
Accessed: 8 June 2022
https://en.wikipedia.org/wiki/Nirmatrelvir/ritonavir
- As of May 2022, the effectiveness among vaccinated people and the effectiveness against long COVID are still unknown
- The co-packaged medication is not authorized for the pre-exposure or post-exposure prevention of COVID-19 or for initiation of treatment in those requiring hospitalization due to severe or critical COVID-19
- The co-packaged medication is not recommended during pregnancy and in women who can become pregnant but who are not using contraception.
- There are no human data on the use of nirmatrelvir during pregnancy related to the risk of birth defects, spontaneous abortions (miscarriage), or adverse outcomes. There are also no human data on the presence of nirmatrelvir in human milk, its effects on milk production or the infant. In pregnant rabbits, a reduction in fetal body weight was observed with systemic exposure 10 times higher than the authorized human dose of the co-packaged medication. A temporary reduction in body weight was observed in the offspring of nursing rats.
- Co-administration with certain drugs may have serious effects and may sometimes be fatal.
COVID antiviral pills: what scientists still want to know
Nature – 10 November 2021
https://www.nature.com/articles/d41586-021-03074-5
- Paxlovid acts by inhibiting an enzyme that’s needed to process some viral proteins into their final, functional form. But the drug is a combination of an antiviral and another drug, called ritonavir, which helps to prevent enzymes in the liver from breaking down the antiviral before it has a chance to disable the coronavirus. Ritonavir, a component of some HIV treatment cocktails, can affect how some other medications are metabolized by the body. A wide range of drugs should not be given with it, including some that are commonly used to treat heart conditions, suppress the immune system and reduce pain.
Three more points about Paxlovid for covid-19
BMJ 2022; 377 :o1397 doi:10.1136/bmj.o1397 Phizackerley D.
https://www.bmj.com/content/377/bmj.o1397
- The evidence for Paxlovid is based on its effects in people who were not vaccinated against SARS-CoV-2. The EPIC-HR study recruited unvaccinated, symptomatic adults with a confirmed diagnosis of SARS-CoV-2 infection within 5 days who were not admitted to hospital and who were at increased risk of progressing to severe illness. Anyone who had received or was expected to receive any dose of a SARS-CoV-2 vaccine was excluded from the study. We do not know how effective the drug will be in a highly vaccinated population.
- The ritonavir element of Paxlovid has a high number of significant drug-drug interactions and the product information contains details of contraindications and warnings for drugs that are affected by Paxlovid.
“Paxlovid (Nirmatelvir/Ritonavir) and Tacrolimus Drug-Drug Interaction in a Kidney Transplant Patient with SARS-2-CoV infection: A Case Report.”
Transplantation proceedings, S0041-1345(22)00286-X. 19 May. 2022, doi:10.1016/j.transproceed.2022.04.015 | Prikis, Marios, and Alexandra Cameron.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9119725/ | PDF
- The 34yo patient developed significant symptoms resulting in interruption of treatment and also acute kidney injury most likely due to toxicity by either the parent FK506 compound and/or its metabolites.
Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19.
N Engl J Med. 2022 Apr 14;386(15):1397-1408. doi: 10.1056/NEJMoa2118542. Epub 2022 Feb 16. PMID: 35172054; PMCID: PMC8908851. Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A, Pypstra R, Rusnak JM; EPIC-HR Investigators.
https://pubmed.ncbi.nlm.nih.gov/35172054/
- See Dr John Campbells video in video section
Rapid relapse of symptomatic SARS-CoV-2 infection following early suppression with nirmatrelvir/ritonavir.
Research Square. 2022. Gupta K, Strymish J, Stack G, Charness M.
https://www.researchsquare.com/article/rs-1588371/v1.
- Was referenced in official NIH Covid treatment document – yet to look at.
Rapid Relapse of Symptomatic SARS-CoV-2 Infection Following Early Suppression with Nirmatrelvir/Ritonavir | April 26, 2022
https://assets.researchsquare.com/files/rs-1588371/v1/48342d2c-b3ea-4228-b600-168fca1fded7.pdf?c=1650977883
- 71-year-old vaccinated and boosted male – rapid resolution of symptoms then viral load & symptoms returned a week later
Pfizer’s Paxlovid reduces COVID risk in seniors regardless of vaccine status – BUT NOT IN YOUNG ADULTS | June 2, 2022 https://wsau.com/2022/06/02/pfizers-paxlovid-reduces-covid-risk-in-seniors-regardless-of-vaccine-status-study/
- Study Finds Pfizer’s Paxlovid Reduces COVID-19 Risk in Seniors Regardless of Vaccine Status, but Not in Young Adults
- Pfizer’s antiviral treatment Paxlovid reduces COVID-19 hospitalization and death rates in vaccinated and unvaccinated patients 65 years and older, according to a new study in Israel conducted during the rise of the Omicron variant of SARS-CoV-2.
- The treatment, however, was not found to prevent severe illness among younger adults, according to research from Clalit Health Services, Israel’s largest healthcare provider.
- The Israeli study, which was published without peer review as a preprint by online platform Research Square, included data from nearly 110,000 participants between January 9 to March 10, when Omicron was the country’s dominant SARS-CoV-2 variant.
- Senior citizens (65 and older) who had no prior immunity reportedly saw an 86% drop in hospitalizations with Paxlovid. Those who had prior immunity also benefited, but at a lower rate of 60%, according to the researchers.
- In patients ages 40-64, however, regardless of their prior immunity, the data showed no significant benefit in reducing hospitalization.
- Regarding mortality, the treatment showed a very high benefit in patients 65 and older—an 81% risk reduction, Clalit researcher Ronen Arbel said.
- Again, there were no observed benefits in younger adults.
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. Paxlovid. 2022 Jan 31. PMID: 35138785.
https://pubmed.ncbi.nlm.nih.gov/35138785/
https://www.ncbi.nlm.nih.gov/books/NBK577815/ - The Safety of Paxlovid in Hemodialysis Patients With Covid-19 (SPHPS)
May 20, 2022
https://clinicaltrials.gov/ct2/show/NCT05366192 - EPIC-HR: Study of Oral PF-07321332/Ritonavir Compared With Placebo in Nonhospitalized High Risk Adults With COVID-19
https://clinicaltrials.gov/ct2/show/NCT04960202
- Pfizer Tweet
“Alongside vaccines, success against #COVID19 will likely require #antiviral treatments for those who contract the virus. We’ve started a Phase 2/3 trial to evaluate a potential oral therapy that will enroll over 2,000 participants infected with SARS-CoV-2: https://on.pfizer.com/376FGpI”
- PAXLOVID™ (nirmatrelvir tablets; ritonavir tablets) has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARS CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death.
- The emergency use of PAXLOVID is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization revoked sooner.
- Pfizer press release (5th November 2021)
- our oral antiviral candidate, … has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations
- Pfizer Shares In Vitro Efficacy of Novel COVID-19 Oral Treatment Against Omicron Variant
- PAXLOVID™ for Post-Exposure Prophylactic Use
- not statistically significant (no significant benefit)
- Pfizer Shares Top-Line Results from Phase 2/3 EPIC-PEP Study of PAXLOVID™ for Post-Exposure Prophylactic Use | Pfizer
- What Is ‘Paxlovid Mouth’? People Report ‘Bitter, Metallic’ Taste After Taking COVID Medication | May 11, 2022
https://www.health.com/condition/infectious-diseases/coronavirus/what-is-paxlovid-mouth-bitter-metallic-taste-side-effects - Paxlovid Rebound: FDA Investigating Reports Of Covid-19 Relapses After Taking Pfizer Antiviral | May 19, 2022
https://www.forbes.com/sites/brucelee/2022/05/19/paxlovid-rebound-some-suffering-covid-19-relapses-after-taking-pfizer-antiviral/?sh=7024abdeb8e4 - Pfizer says COVID treatment Paxlovid fails to prevent infection of household members | Reuters
https://www.reuters.com/business/healthcare-pharmaceuticals/pfizer-says-covid-treatment-paxlovid-fails-prevent-infection-household-members-2022-04-29/ - Covid-19 cases that return after Paxlovid antiviral treatment puzzle doctors – CNN
https://edition.cnn.com/2022/04/27/health/paxlovid-covid-rebound/index.html - Paxlovid Mouth Is Real—And Gross – The Atlantic
https://www.theatlantic.com/health/archive/2022/05/pfizer-paxlovid-covid-pill-side-effects/629772/
Paxlovid is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious and/or life-threatening reactions. Paxlovid is also contraindicated with drugs that are potent CYP3A inducers where significantly reduced nirmatrelvir or ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance. Paxlovid cannot be started immediately after discontinuation of a potent CYP3A inducer, due to the delayed offset of the recently discontinued CYP3A inducer. Paxlovid now has 13 PAGES of KNOWN Drug-Drug interactions in the TGA Licence Agreement. Too many to list in this post and I’m not sure if I’m even “allowed” to list them! See: Pages 7-19.. (04) Paxlovid® (nirmatrelvir and ritonavir) Pharmaceutical Benefits Scheme Factsheet – Updated February 2023 https://www.pbs.gov.au/publication/factsheets/covid-19-treatments/Factsheet-paxlovid-nirmatrelvir-and-ritonavir-Feb-2023.pdf (05) AUSTRALIAN PRODUCT INFORMATION – PAXLOVIDTM (nirmatrelvir/ritonavir tablets) https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2022-PI-01049-1&d=20220404172310101&d=20230226172310101 (06) TGA – Paxlovid – Product and Consumer Medicine Information Licence https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2022-PI-01049-1&d=20220404172310101

Site Notifications/Chat:
- Telegram Post Updates @JourneyToABetterLife (channel)
- Telegram Chatroom @JourneyBetterLifeCHAT (say hi / share info)
- Gettr Post Updates @chesaus (like fakebook)
Videos:
References[+]
Truth-seeker, ever-questioning, ever-learning, ever-researching, ever delving further and deeper, ever trying to 'figure it out'. This site is a legacy of sorts, a place to collect thoughts, notes, book summaries, & random points of interests.
DISCLAIMER: The information on this website is not medical science or medical advice. I do not have any medical training aside from my own research and interest in this area. The information I publish is not intended to diagnose, treat, cure or prevent any disease, disorder, pain, injury, deformity, or physical or mental condition. I just report my own results, understanding & research.









![[Police Standing up] Craig Backman – Former VIC Police](https://pennybutler.com/wp-content/uploads/2021/12/CraigBackman.jpg)

