COVID-19 Vaccines are Experimental
Research valid as at 30 September 2021
Index of Downloadable Refs:
“List of Verifiable References”
DO NOT LINK DIRECTLY TO THIS WEBSITE.
DOWNLOAD THE FILES SEPARATELY FROM THE POST ABOVE.
Covid-19 Vaccines are defined as “experimental” by their Manufacturers
Pfizer clinical trial – page 46

The long-term effects are not known and cannot possibly be known until a “long time has passed”.
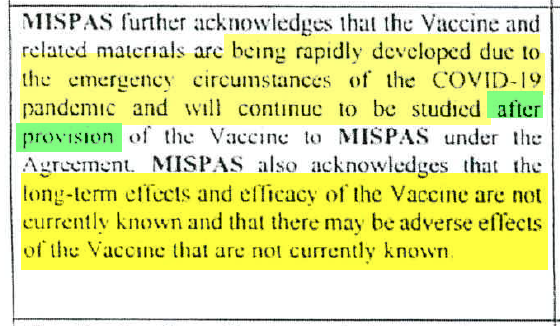
Pfizer claims vaccine is “Aspirational in nature, subject to Significant Risks, may not be successful, being rapidly developed due to pandemic, will continue to be studied after provision, and that the long-term effects, efficacy, and adverse effects are not known“
The Dominican Republic contract, obtained via a Freedom of Information request, states:
“long-term effects and efficacy of the Vaccine are not currently known and there may be adverse effects of the Vaccine that are not currently known.
… efforts to develop & manufacture the Vaccine are aspirational in nature…
… subject to significant risks and uncertainties.
… the Vaccine may not be successful….”
….being rapidly developed due to the emergency circumstances of the COVID-19 pandemic and will continue to be studied after provision…
… long-term effects and efficacy of the Vaccine are not currently known and there may be adverse effects of the Vaccine that are not currently known.
Pfizer Supply Agreement Contract – Dominican Republic – obtained via a Freedom of Information Request
https://www.keionline.org/misc-docs/Pfizer-DominicanRepublic-Vaccine-Term-Sheet-19Jan2021.pdf
Note: All attempts to get a copy of the Pfizer contract by Freedom of Information requests (and by members of parliament) in Australia have thus far been unsuccessful due to “containing confidential and commercially sensitive information“. – https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/covid-19-vaccine-government-response/australias-vaccine-agreements
COVID-19 Vaccines implement new technology
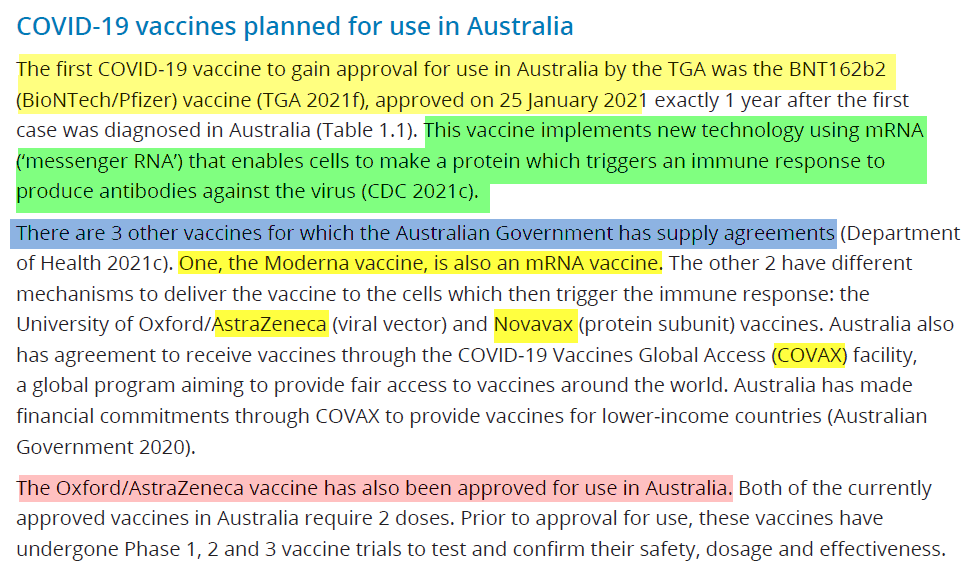
Australian Government: Australian Institute of Health and Welfare
https://www.aihw.gov.au/reports/burden-of-disease/the-first-year-of-covid-19-in-australia/summary
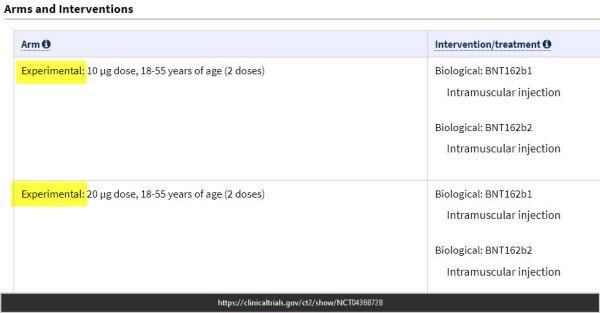
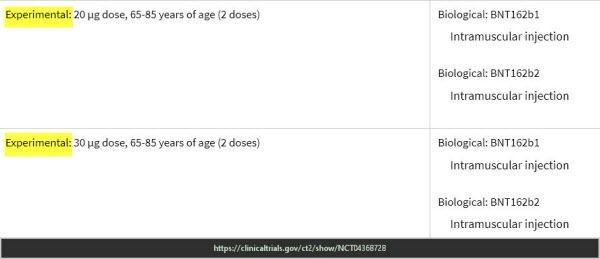
Pfizer Trial – End points keep changing & experimental doses
ClinicalTrials.gov Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals https://clinicaltrials.gov/ct2/show/NCT04368728
First Trials began just 66 days after RNA uploaded to a GenBank database
The vaccines were fast-tracked and the first US clinical trials began just 66 days after the RNA sequence was uploaded to the GenBank database. (P.15)
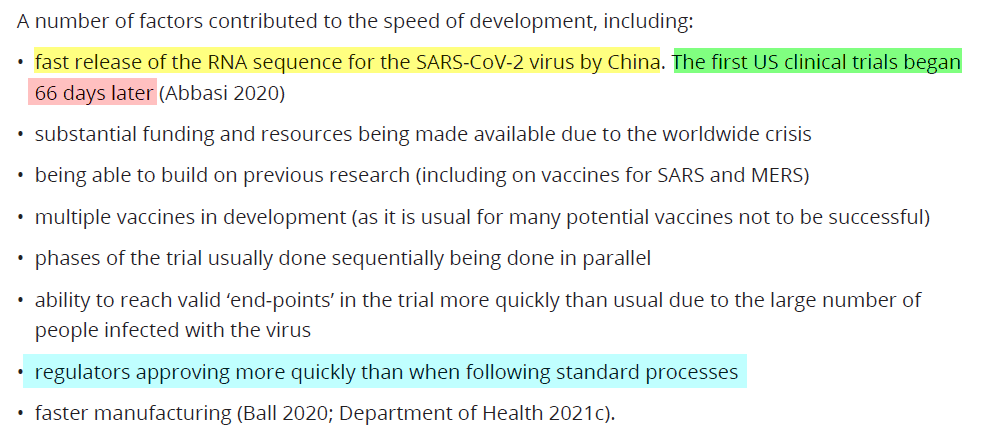
The vaccine manufacturers developed Vaccines, and Tests were created, based on an RNA sequence uploaded to a database. Every vaccine, test, and trial was developed based upon these GenBank uploads.
‘January 2021, the SARS-CoV-2 genome sequence was published to GenBank: MN908947.1
Which had reportedly been collected by the team from the Wuhan Central Hospital and the WHCDC.
Wuhan seafood market pneumonia virus isolate Wuhan-Hu-1, complete genome
https://www.ncbi.nlm.nih.gov/nuccore/MN908947.1
Australian Government: Australian Institute of Health and Welfare
https://www.aihw.gov.au/reports/burden-of-disease/the-first-year-of-covid-19-in-australia/summary

Site Notifications/Chat:
- Telegram Post Updates @JourneyToABetterLife (channel)
- Telegram Chatroom @JourneyBetterLifeCHAT (say hi / share info)
- Gettr Post Updates @chesaus (like fakebook)
Videos:







![[Australia] What’s In The Pfizer Vials?](https://pennybutler.com/wp-content/uploads/2022/12/DavidNixonLights-777x437.jpg)
