Vaccines Failed to Disclose Absolute Risk Reduction to Exaggerate Efficacy

Epidemiologist reveals how the Moderna and Pfizer COVID-19 vaccine trials grossly exaggerated the usefulness of the injection and represents the “Worst Miscalculation in Human History.” Dr. Ron Brown, PhD. (05)
From the Conclusion: “A critical appraisal of phase III clinical trial data for the Pfizer/BioNTech vaccine BNT162b2 and Moderna vaccine mRNA-1273 shows that absolute risk reduction measures are very much lower than the reported relative risk reduction measures. Yet, the manufacturers failed to report absolute risk reduction measures in publicly released documents.
As well, the U.S FDA Advisory Committee (VRBPAC) did not follow FDA published guidelines for communicating risks and benefits to the public, and the committee failed to report absolute risk reduction measures in authorizing the BNT162b2 and mRNA-1273 vaccines for emergency use. Such examples of outcome reporting bias mislead and distort the public’s interpretation of COVID-19 mRNA vaccine efficacy and violate the ethical and legal obligations of informed consent.” (06)
An interview between Trial Site News & the Author of the paper, Dr. Ron Brown communicates what the paper means. (07)
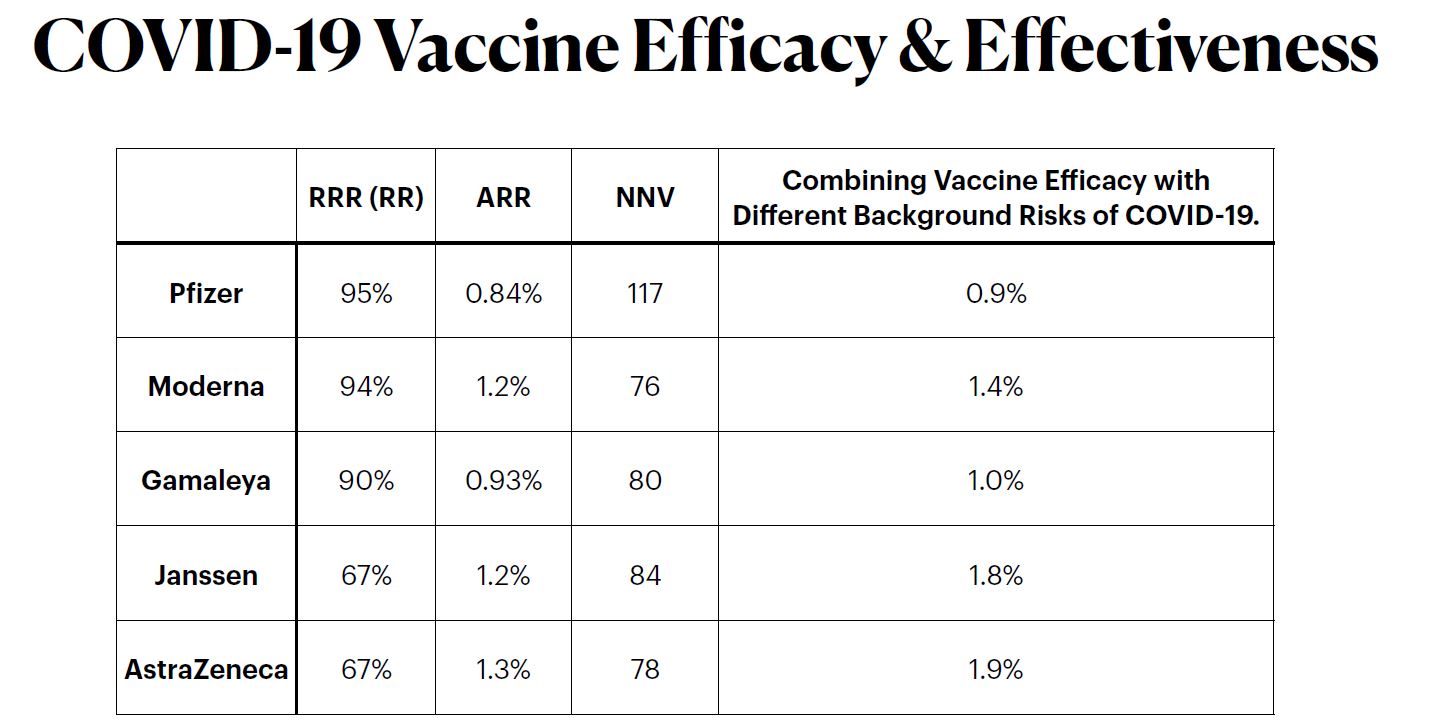
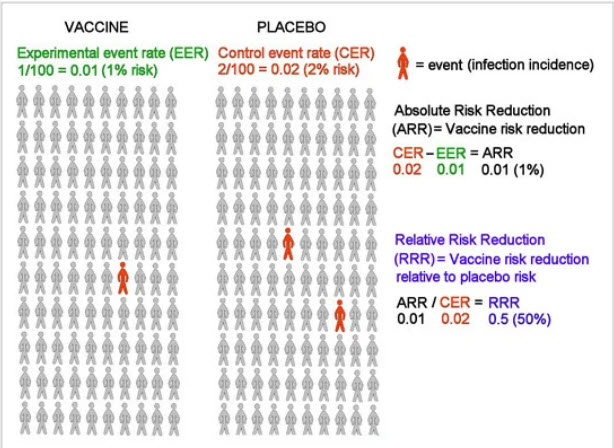
Based on data reported by the manufacturer for Pfzier/BioNTech vaccine BNT162b2, this critical appraisal shows:
relative risk reduction, 95.1%; 95% CI, 90.0% to 97.6%; p = 0.016;
absolute risk reduction, 0.7%; 95% CI, 0.59% to 0.83%; p < 0.000.For the Moderna vaccine mRNA-1273, the appraisal shows:
relative risk reduction, 94.1%; 95% CI, 89.1% to 96.8%; p = 0.004;
absolute risk reduction, 1.1%; 95% CI, 0.97% to 1.32%; p < 0.000.Unreported absolute risk reduction measures of 0.7% and 1.1% for the Pfzier/BioNTech and Moderna vaccines, respectively, are very much lower than the reported relative risk reduction measures.
Reporting absolute risk reduction measures is essential to prevent outcome reporting bias in evaluation of COVID-19 vaccine efficacy.
Source: Abstract: Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials (08)
Some people may point out that 1% of a million vaccinated people are still 10,000 prevented symptomatic infections. Fair enough; then report a 1% reduction and see how many people are still interested in getting the vaccine. Furthermore, there is no reliable evidence that even a reported 1% reduction is valid. Regardless if you are provax or antivax or are undecided, you have a right to all the facts to inform your personal opinion and choice.
The New England Journal of Medicine (09) | CDC – Pfizer (10) CDC – Moderna (11).
Why Relative Risks are Misleading
This video (not about vaccines) explains why reporting “just relative risks” without including “absolute risks” is misleading. (12)
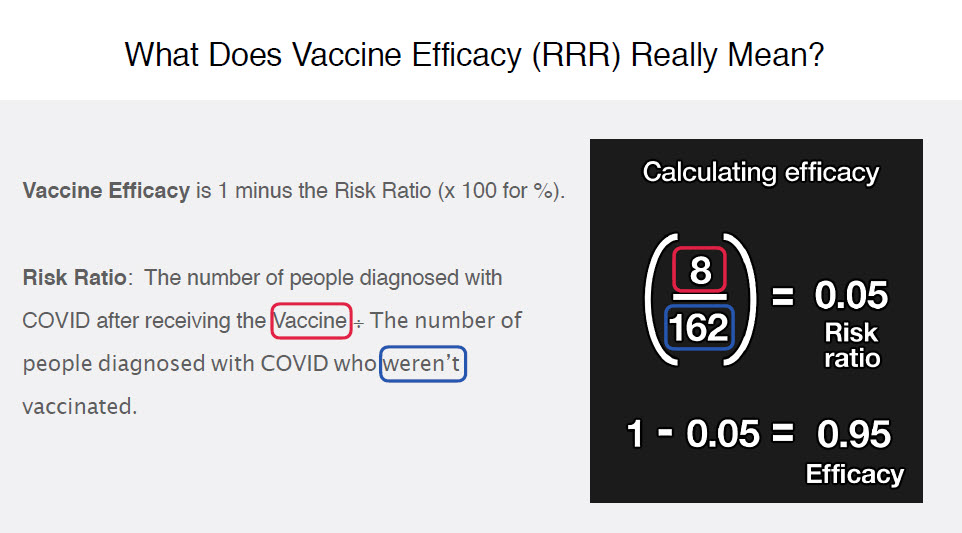
He mentions a tool at the end of the video, called the !RealRisk tool by the University of Cambridge (13) I played around with it by plugging in the data from the clinical trial, and it concurred that out of 100 people, the absolute risk reduction is about 1 in 100. I interpret that as meaning that out of 100 people who take the vaccine, only 1 would have reduced serious COVID symptoms? But if you plug in the reported “relative risk” data of 95.1%; with the “90-97% confidence level” (that they stated in the clinical trial outcomes), the data magically makes the vaccines look “miraculously very favourable” (suddenly 67 out of 100 wouldn’t develop serious COVID symptoms). Huge difference and something we probably should be informed about before taking a risk with experimental vaccines on a global scale, especially true because of the suppressed COVID-19 vaccine-victims data (14) – do we want to risk blood clots and permanent vaccine damage for a ‘1%’ potential ‘protection’?
Now – before I get carried away with any uneducated assumptions I might be making – I don’t trust my own data interpretation because this is not my field – I’m just a person trying desperately to understand what all things COVID means, and there’s nothing worse than “people like me who don’t understand the data, coming to conclusions about data they don’t understand” – so I’m looking for more of those who “do understand what this means” who can reliably explain it.
On TrialSiteNews again, Dr Ron Brown’s ‘response to the argument about absolute risk reductions’ stated: (15):
- The Pfizer/BioNTech vaccine’s absolute risk reduction is 0.7%, and the vaccine efficacy or relative risk reduction (RRR) is 95.1% (16)
- Published results from the Pfizer/BioNTech phase 3 clinical trial covered the period from May 2020 to November 2020, having a trial length of 6-7 months; more than just “a few weeks” claimed by the Medscape.com commentary. (17)
- Let’s double the Pfizer/BioNTech phase 3 trial from seven months to 14 months, and also assume that cases in the vaccine group and the placebo group double. Under these circumstances, as the author indicated, the ARR will now double from 0.7% to 1.4% while the RRR remains the same at 95.1%. But how much has the ARR improved compared to the RRR? Not much. Let’s double the cases and trial time again. The ARR at 28 months is now 2.8% compared to the RRR of 95.1%…still pretty low. Get the point? The effect of length of time on the ARR compared to the RRR is relatively meaningless from a clinical and public health point of view.
- At 56 months the ARR is 5.6%; 112 months: 11.2%; 224 months: 22.4%; 448 months: 44.8%; 896 months: 89.6%…it would take over 75 years for the ARR to reach the RRR level under assumed conditions of consistent vaccine efficacy!
- The more important point is that the relative risk measures, ARR and RRR, are both valid only for the actual length of the clinical trial. One cannot assume that the effectiveness of the vaccine in the “real world” would continue at the same efficacy rate beyond the trial length without supporting evidence.
- Pharmacotherapies have limited real life effectiveness over time, including vaccines for influenza-like illnesses. Pharmaceutical companies rely on time-limited benefits to promote annual sales of seasonal influenza vaccines and booster shots.
- In conclusion, the argument in the Medscape.com commentary, that a vaccine’s ARR increases indefinitely with increasing length of time, is invalid because it assumes vaccine efficacy will continue at the same rate beyond the clinical trial length, without providing any strictly controlled experimental evidence to support this assumption.
There are ‘letters to the editor‘ querying the Pfizer trial data. Excerpts below: (18)
In their article, however, questionable results are reported in Table 3. In each trial group, the sum of the number of cases across age groups (9 in the vaccine group and 186 in the placebo group) does not equal the overall number of cases (8 and 162, respectively). The reasons for the discrepancy are not clearly explained in the article. (19)
To the Editor – Jean-Noel Vergnes, D.M.D., Ph.D.
(Table 3 is listed in this post under ‘side effects’)
The percentage of Covid-19–positive patients in whom severe illness developed was (9 of 162 patients) in the placebo group and (1 of 8 patients) in the vaccine group. Thus, the preliminary data do not appear to support the conclusion that this vaccine offers protection against severe Covid-19 illness or alleviate the theoretical concern over vaccine-mediated disease enhancement, given that the percentage of Covid-19–positive patients in whom severe illness developed was significantly higher in the vaccine group than in the placebo group. (20)
To the Editor – Xiang Wang, Pharm.D.
Response by the Authors:
We would like to clarify that it is not appropriate to use the proportion of Covid-19–positive patients in whom severe disease developed to assess vaccine protection against severe Covid-19. Protection against severe illness is an integrated effect of reducing the chance that any Covid-19 symptom will develop and reducing the risk that severe symptoms will develop after infection. The estimation of vaccine efficacy against severe illness should be based on the incidence of severe illness in the total study population. (21)
Response by the authors
Does that sound right to you?
Dr.Been says reporting Relative Risk data is not misleading:
Dr. Been takes a different approach to whether the “Relative Risk” data is misleading – he pretty much says it’s not: (23) (24) (25)
From the comments on the video which are mostly thanking him for explaining it, and, although grateful for the explanation of how ‘relative risk’ is calculated, most do not agree with leaving out the absolute risk data, and bring up the following points:
- Relative Risk Reduction (RRR) – assumes you WILL get COVID. And how much better you will fair AFTER getting COVID. Absolute Risk Reduction (ARR) – which is real world during the time of the trials. You have a VERY LOW chance of getting COVID. You will probably NOT get COVID.
ARR brings in this real world scenario of a 3rd, and most likely, scenario (at least during the case counts during the trials). RRR is binary: You get COVID is a given. Here’s is your risk reduction after you take the vaccine vs not taking the vaccine.
ARR factors in 3 scenarios: The 3rd scenario is you DON’T get COVID, with or without the vaccine. So it is best to assess your city / county / state and wherever you frequent case counts and outbreaks. If the cases are low around where you spend your time, or low in general across your country, you have no real-world benefits to taking the vaccine because you have a minimal chance of getting COVID in the first place. - The problem is definition and consistency of what they (vaccine manufacturers) are measuring: when they say the INT group or the CTR group got C19, how did they establish that anyone got C19? was it PCR test? was there variability in measurement? Also, the general public ought to be better informed in as many ways as possible so that we understand perfectly and it becomes common knowledge how to understand RRR in the context of ARR.
- RRR is qualitative. ARR is quantitative. Media and so called experts using the the high efficiency rates (which you admit is qualitative -RRR) , to falsely imply that if you take the vaccine, 95% of the population will not get the infection.
- Relative risk is how Big Pharma turns 0.7 people out of hundred benefiting from the vaccine into “94% efficacy.”
- If you look deeper into the phase 3 data you will see that there were no reported covid related deaths in this trial of over 36000 individuals. As well as a very small percentage of covid positive tests. 170 total positive cases 0 deaths. 119 as the number needed to treat for 1 person to benefit on average. Now compare the study death rate and positive test rate with real world numbers and look at the huge disparity.
- If we tell people that one out of 100 vaccinated people can be prevented to have the disease, I really doubt a lot of people will jump in to get vaccinated, not to mention the side effects. The vaccine companies didn’t provide people the ARR for a good reason.
- If I tell you that I can reduce your risk by 90%, you cannot make a proper decision if you do not know what your absolute risk is without the treatment. Why do you think the Pharma does not comply with the FDA guideline ? Much better selling argument to say Relative Risk Reduction is 90 % than adding that the absolute risk reduction is 0,7 % for Moderna or 1,1 % for Pfizer by way of illustration.
- In my view, I would rather stick to absolute risk reduction as the data demonstrates from the get go (without intervention) the risk is very low 0.88%. Vaccine intervention is not required.
- Something to consider: the number used to represent the risk of contracting the virus in the control group may be seriously flawed if they are using the PCR test. This value is easily manipulated by altering the cycle threshold. The question is: Are the researchers being transparent about the CT used for each group? Could there be any financial motivation to use different parameters in each arm of the study? The pharmaceutical industry does not have a good track record of integrity in self-reported data when it comes to justifying their bottom line.
- Thanks for the explanation, and i understand the importance of publishing the relative risk number. But surely publishing the absolute risk reduction alongside would give people the knowledge to decide if they want the medication or not. Eg. Why would i take medication / vaccine for a virus if the absolute risk reduction is .8%… most medications have side effects, they might be of benefit for one thing but cause other problems… that way people are empowered to calculate the risk for themselves.
- Nice. Except if the vaccine had no side effects 95% relative risk reduction would be great. When the side effects of an intervention is significant, the number needed to vaccinate minus 1 says how many people get the side effects with no benefit. 10000÷84 . More than 110 people vaccinated will experience the side effects without any benefit.
“The Math”
Another video explains “the math” behind the reports of 95% efficacy for the Pfizer vaccine: (26)
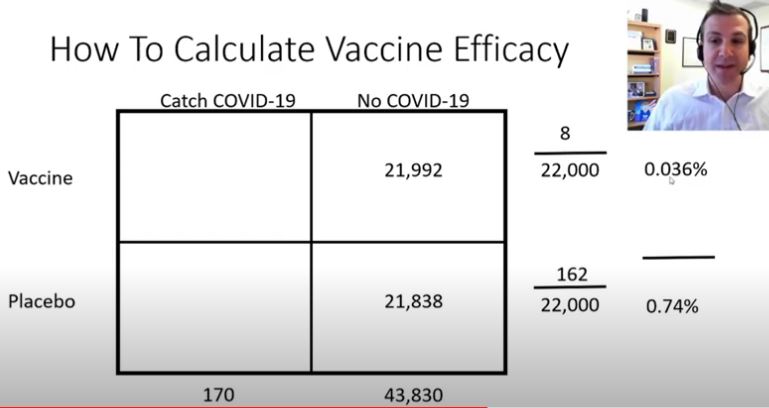
Of which he explains that 22,000 were in each group (Vaccine vs Placebo). That 8 ‘got sick’ in the vaccine group and 162 ‘got sick’ in the placebo group, and how that calculates to a 95% efficacy.
Wait… only 5 people hospitalized?
Am I the only one that watched this video whose first thought was… wait a second. 44,000 people, only 170 got sick, and only 5 people got severe-covid?
When the media broadcasts the 95% efficacy number, which is only a “relative risk” number based on those in the study that didn’t get covid, they fail to mention that almost all of the people in the study didn’t get covid, and that they are basing a 95% efficacy number on just 5 people getting severe covid. They ARE being deceptive if they don’t include the actual numbers, because people are making a risky decision based on 5 people who got severe-covid.
Pfizer Vaccine Efficacy Explained
F. Perry Wilson, MD 19 Nov 2020
The media is the ‘trusted-source’ for the ‘non-researchers’ to get their information. Most ‘normal everyday people’ are going to actually interpret that headline to think that having the vaccine will give them 95% protection against getting severe COVID, but you cannot translate 5 people out to the whole world. (This kind of conversation is ‘normal’- People read/watch the news & literally only take in the headlines and parrot it to others as fact – without understanding what it means).
Now I need to know what they determine “sick” to be in the trials. What is ‘sick’? Sick as in “requires medical intervention sick”, or sick as in “I’m going to take the day off work”, or sick as in “I have a bit of a headache/sniffle”? (How do they determine exactly what COVID-19 symptoms look like when they are so similar to the flu – how do they “determine” that data exactly, blood clots and lung failure? or “feeling a bit unwell”. How do they know the difference between COVID & the normal flu?
Update (next day) 21 Jun 2021: Found the ‘what determines “sick” data’ for Pfizer (27) (I’m still annoyed that I have to dig for this), I’m also finding discrepancies in the numbers which has added further confusion, making me wonder if these are completely different trials or whether hundreds of people were dropped from the end-result – if anyone understands why, please reach out – but this is another reason if you are creating these ‘explainer videos’ to include links to the specific trials you are ‘explaining’:
A total of 43,548 participants underwent randomization, of whom 43,448 received injections: 21,720 with BNT162b2 and 21,728 with placebo.
Results section
Adverse event analyses are provided for all enrolled 43,252 participants, with variable follow-up time after dose 1
Adverse events section
See why I’m confused with numbers?
- Pfizer Efficacy Trial ‘Math’ video says 44,000
- Dr Bean’s Video refers to the Pfizer Phase III trial as 18,198 in the vaccine group & 18,325 in the placebo group (36,523)
- Results/introduction of the “trial I’m looking at which is referred to as Pfizer Phase 2/3” says 43,548
- Adverse events ‘for all enrolled’ section says 43,252.
(Are we all referring to completely different trial data with similar participant numbers or a different set of result-reporting or we all reporting different ‘results’ from the same trial data? please help me understand this discrepancy? None of the videos or reports link to the trial data they are reporting on!
“Sick” data
The first primary end point was the efficacy of BNT162b2 against confirmed Covid-19 with onset at least 7 days after the second dose in participants who had been without serologic or virologic evidence of SARS-CoV-2 infection up to 7 days after the second dose;
The second primary end point was efficacy in participants with and participants without evidence of prior infection.
Confirmed Covid-19 was defined according to the Food and Drug Administration (FDA) criteria as the presence of at least one of the following symptoms:
- fever
- new or increased cough
- new or increased shortness of breath
- chills
- new or increased muscle pain
- new loss of taste or smell
- sore throat
- diarrhea
- vomiting
- combined with a respiratory specimen obtained during the symptomatic period or within 4 days before or after it that was positive for SARS-CoV-2 by nucleic acid amplification–based testing, either at the central laboratory or at a local testing facility (using a protocol-defined acceptable test).
Major secondary end points included the efficacy of BNT162b2 against severe Covid-19. Severe Covid-19 is defined by the FDA as confirmed Covid-19 with one of the following additional features:
- Clinical signs at rest that are indicative of severe systemic illness;
- Respiratory failure;
- Evidence of shock;
- Significant acute renal, hepatic, or neurologic dysfunction;
- Admission to an intensive care unit;
- or Death.
Did they use PCR tests – what was the cycle threshold used for each person tested? How can you determine 95% efficacy from only 8 people getting ‘sick’ when it seems the majority of people are not getting ‘sick’ across the entire trial and you haven’t mentioned whether any of the others were ‘positive’ or not – were only those ‘with symptoms’ tested?
Update (next day) June 21st 2021, Answer: (28)
Ok, what is this protocol-defined acceptable test please? Since this is a big debate from the beginning of the entire COVID-19 pandemic as to the accuracy of the various tests and whether they should even be used or relied upon.
And in the Moderna trial (29) which had over 30,000 volunteers, 196 had ‘symptomatic covid-19’ (‘at least 14 days after they received their second shot’*), 185 from the placebo. Of which, 10 people were hospitalized (1 vaccinated, 9 placebo)
Can I ask what happens to those who got covid less than 14 days after receiving the first or second jab? Enquiring minds would like to know – me – I would like to know. I found first jab info (below) but still want to know about ‘less than 14 days’ after first shot – is that data deleted, counted, included somewhere? (Is that the reason why there are discrepancies with the numbers?)
Anyway, we have 30,420 + 44,000 phase 3 trial participants, and 461 (236+225) + 170 who ‘got sick’, and out of 74,420 people, we only have 10 + 5 hospitalizations? I feel like I’m taking crazy pills here – but how are we justifying vaccines out to ——————- THE ENTIRE GLOBE ——————- based on this? How is it front-page news to encourage ‘everyone’ to get vaccinated, based on data like this? What am I not seeing here, because this is not enough proof that the vaccines are extremely effective?
You’re basically telling the world that its 95% effective at preventing severe covid, on 170 + 236 symptomatic cases of which only 15 people (placebo+vaccinated) got severe covid (and almost 75k who didn’t even get covid). This is ‘roll the dice’ numbers – not proof of anything, right? 15 people – are you kidding me. Am I also biased myself because I was in a group of 75k people who had adverse affects from the covid vaccines of which there was post after post of heart-breaking stories of deaths of loved ones, ICU trips, strokes, paralysis, bell’s palsy, etc. and severe adverse injuries from the vaccines themselves?
What ‘side effects’ did those in the vaccine group get in comparison to the ‘placebo’ group?
Why do I… as a ‘lay person’, have to look up and download trial data to find out this information – why are you not including this when you present the data?
Where’s the table that shows the blood clots, hives, swelling, neurological issues, tremors & uncontrollable shaking, facial numbness, burning sensations, myocarditis, erythromelalgia, bell’s palsy, spinal cord inflammation, disorientation, loss of memory, brain fog, aggression, night terrors, loss of vision, complete hearing loss, mouth sores, respiration issues, cardiovascular issues, muscle fatigue, menstrual irregularities, spontaneous miscarriages, multi-system organ failure, strokes, and more that are reported elsewhere? (30) (31) (32)
I’m saying this because the vaccine(s) safety data should be reported along with the absolute risk – people need to know – before they hold their arms out – exactly what their chance of getting covid is (and it seems that it’s not very high at all in a lot of places), the likelihood of getting ‘severe’ covid (extremely low in these trials), and what risks they are taking by having a vaccine they may not even need (shrugged-off/downplayed/censored), and whether it’s even safe one way or another. We need you to stop pushing the vaccines when the safety data of the vaccines themselves are obscured, and the numbers getting severe covid in these trials – placebo or not – are so very low, so they shouldn’t be used as proof these vaccinations are worth the risk of the adverse affects of the vaccines themselves. (If you’re inflating the numbers to assume that ‘everyone is going to get severe covid’ if they get covid at all, as a determining factor in this vaccine push).
Update (next day) 21 Jun 2021: Found the “Pfizer” adverse effects data’ (33) – (will seek Moderna data next & update – Ok update: cannot locate Moderna phase 3 trial results because the official trial 3 page (34) has no results posted yet, and there is only that preliminary review (35) that doesn’t include the data, and the Moderna site (36) itself which only has a summary, please correct me if I’m wrong of if you have located the data:
Adverse event analyses are provided for all enrolled 43,252 participants, with variable follow-up time after dose 1 (Table S3)
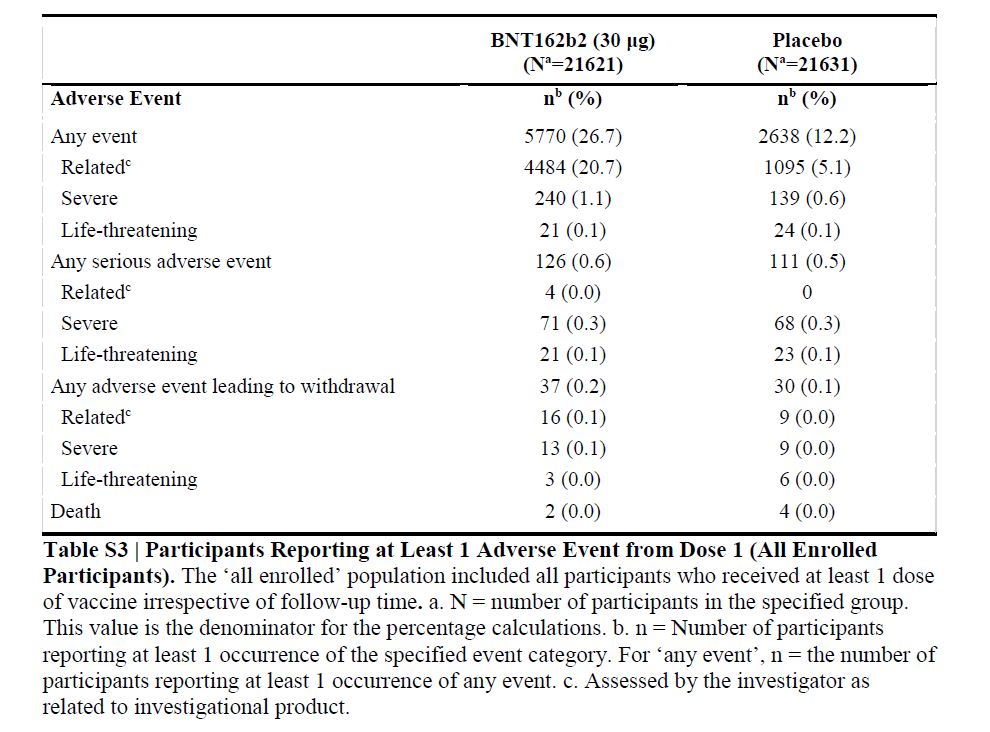
More vaccine recipients than placebo recipients reported any adverse event or a related adverse event.
64 vaccine recipients (and 6 placebo recipients) reported lymphadenopathy.
4 related serious adverse events were reported among vaccine recipients (shoulder injury related to vaccine administration, right axillary lymphadenopathy, paroxysmal ventricular arrhythmia, and right leg paresthesia).
2 vaccine recipients died (one from arteriosclerosis, one from cardiac arrest)
4 placebo recipients (two from unknown causes, one from hemorrhagic stroke, and one from myocardial infarction).
No deaths were considered by the investigators to be related to the vaccine or placebo.
No Covid-19–associated deaths were observed.
Report says: “About 50% of participants receiving mRNA-1273 experienced moderate-to-severe side effects — such as fatigue, muscle aches, joint pain and headache”. (38) Does this feel like it’s being deliberately down-played to anyone else? Half the people got ‘moderate to severe side effects’, but they only mention the moderate-sounding-ones that wouldn’t be listed as ‘severe’ in anyone’s determination, so what are the ‘severe’ ones they are leaving out of this report? I’m annoyed with the ‘peer-review’ process too – (since it’s anonymous and you can never find out who did the review and what links they have to the authors/vaccines).
Please – all of you – who are trying to prove something one way or another with your “this is how it works” explainer videos & reports – stop fucking around with these kinds of ‘basic/unsubstantial’ explanations and give us the actual data we need to determine the risks. You need to stop focusing on that bullshit 95% number – because if it’s based on the trial data above, then it’s based on “air” -this pie-in-the-sky 95% number is based on an assumption that not only will ‘everyone get covid’, but that “everyone” who gets COVID will get “SEVERE covid” which we already know not to be true, but their trials also re-validate that considering only 15 people had severe COVID symptoms.
And when they get vaccinated, not only are any severe adverse reactions to the vaccines obscured, they still have to be quarantined and wear masks, because they can still “spread the disease”. Clearly – that 95% number is a publicity stunt / headline-grabbing marketing ploy at best. You need to go into way more detail when presenting data that is meant to explain the true efficacy and true risk:
What is the actual risk of getting “severe” covid
vs the risk of adverse side effects of the vaccine(s)
vs the risk of potential permanent damage (either way).
If you leave out the other ‘risk factors’, or what the terms mean, you are the reason we don’t trust the biased data to begin with. I don’t know exactly what we should be knowing, but I expect those who are data-mining to understand/explain the risks, should have some kind of data that looks a little more like this:
Data I’d like to see compared:
“Fake” Table – this is the kind of information you need to give us to make an ‘informed decision’ about the risks.
| Vaccine: | Placebo | AstraZeneca | Pfizer | Novavax |
|---|---|---|---|---|
| # | 22,000 | 22,000 | 22,000 | 22,000 |
| Tested Positive CT <25 | ? | ? | ? | ? |
| Antibodies | ? | ? | ? | ? |
| Deaths | ? | 258 | 150 | ? |
| Hospitalization | 4 | 20 | 6 | ? |
| Stroke | 9 | 271 | 42 | ? |
| Blood Clot | 3 | 118 | 4 | ? |
| Bells Palsy | 7 | 414 | 269 | ? |
| Side Effects > 3 days | ? | ? | ? | ? |
| Side Effects > 2 weeks | ? | ? | ? | ? |
| Side Effects > 2 months | ? | ? | ? | ? |
| Permanent Disability | ? | ? | ? | ? |
| Miscarriage | 12 | 123 | 110 | ? |
| Menstrual Disorder | 28 | 1892 | 708 | ? |
| Dyspnoea | 109 | 6071 | 1956 | ? |
| Tremors | ? | 9178 | 893 | ? |
| Paralysis | 2 | 272 | 284 | ? |
| Loss of consciousness | 8 | 936 | 258 | ? |
…and after you have all that data, then you have something that you can use to explain it to the rest of us why you think it’s worth injecting something so new into our most vulnerable and without any known data for long-term issues – on our children! Do I want to play Russian roulette with the ‘other’ risks that you are deliberately omitting from your research, for a 1/100 chance that ‘if’ I get COVID, I ‘might’ avoid severe COVID? Especially when there are way less risky treatments available should ‘severe covid’ be a reality.
See also: Will covid-19 vaccines save lives? Current trials aren’t designed to tell us BMJ 2020;371:m4037 (39)

Site Notifications/Chat:
- Telegram Post Updates @JourneyToABetterLife (channel)
- Telegram Chatroom @JourneyBetterLifeCHAT (say hi / share info)
- Gettr Post Updates @chesaus (like fakebook)
Videos:
References





![[ICU Nurses] All who died from COVID should be considered Murdered](https://pennybutler.com/wp-content/uploads/2022/02/NHCSBoardofEdu-MorganWallace-777x437.jpg)