BMJ: Unclean Data in Vaccine Trials (Pfizer & FDA should be investigated & Rollout Halted)
A recent published paper on the Pfizer Vaccine Trials and FDA has FINALLY awoken some of the doctors who have been calling people “antivaxxers” or “conspiracy theorists” instead of taking more of an “investigative journalistic” approach and checking their claims. You cannot blindly trust pharma-funded corrupt science journals (01). Recently, those with influence are starting to realize that ‘critical thinkers’ haven’t been lying or alarmist over nothing and are becoming more aware of the screwy things going on with these big pharmaceutical companies. Maybe now, we can get the truth to the general population. I hope those in our government agencies and committees who are not corrupt start investigating this too and stop blindly “trusting the journal publications conclusions” at their word and really re-review the data, the companies, and the corruption. I really hope this is the start of a turn-around for the corruption that has infiltrated our science (02), education and health communities for decades.
This latest paper has woken up many:
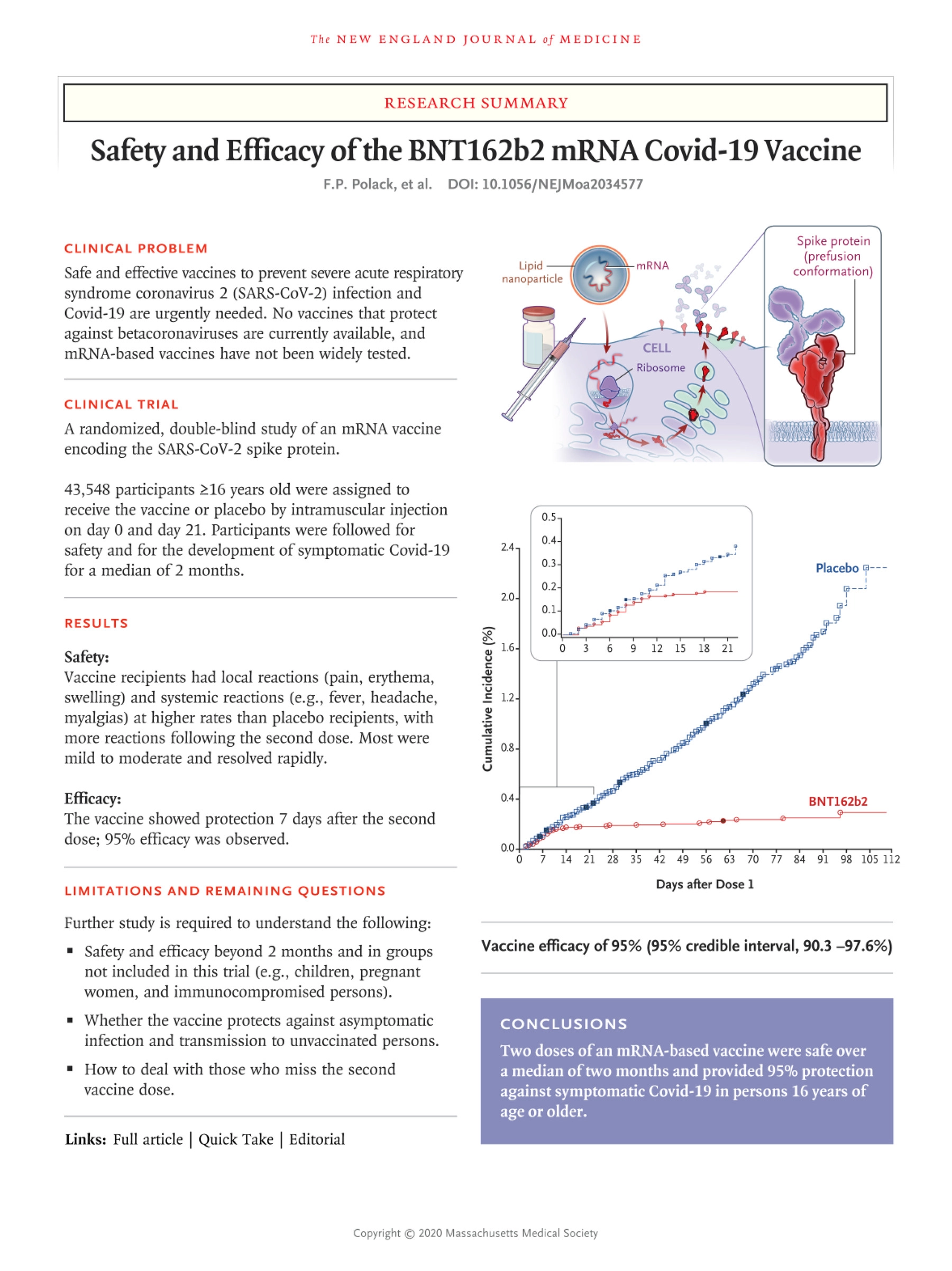
Referring to this Pfizer Clinical Trial
The same trial that showed 95% efficacy and same trial that got the FDA approval, and same trial that the entire world’s health authorities have used as a bases for their approval.
BMJ Interview (View on BMJ)
Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial BMJ 2021;375:n2635 – November 2, 2021 (04)
Main Points:
Screenshots taken from Dr John Campbell’s video:
- Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial (05)
- Screenshots taken from Dr John Campbell’s video (06)
- Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine <– Pfizer trial in Question (The Trial that got the vaccine FDA Approval with 95% effectiveness. (07)
Video’s about this report:
Finally Pro-Vax Influencer-Doctors are realizing that Pfizer Science is flawed – that all is not well in the land of cherry-picked “Science”. Thank you – especially DrBeen – I knew you were good guy – but you’ve also been calling people “antivaxxers” when they’ve tried to reach out to you to get you to investigate and download the actual raw data rather than taking them or trusting their “conclusions” – when someone points out that the science is corrupt – we need to not make assumptions that they are just “conspiracy theorists” – most people labelled with this derogative term are well-studied and are able to see-through things that others may take for granted as ‘fact’ just because it’s in a peer-reviewed journal.
This is how we start to fix the corruption – when doctors, health scientists, and committees who have been trusting the journal publications at face-value without realizing these giant pharmaceutical companies have been fudging the data for decades, start looking beyond the carefully-crafted science manipulation There is hope that the truth will reach the people now.
The corruption in Science, Education, and Health needs to end. And if any of you doctors that I’ve been reaching out to want another tip – please look at what’s been “held up” in the pre-print servers to see what “science” they aren’t allowing to be approved. We need you to call this out and let people know they are deliberately suppressing ‘science’ if it doesn’t fit the current agenda.
DrBeen: YouTube
An investigational report published in the British Medical Journal (theBMJ) presents the faulty trial methods and unclean data from one of the Pfizer trial partner companies. Ventavia research group is at the company with these gaps. Let’s review the report.
- Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial (08)
- Pfizer’s Power – Full Report – Oct 19, 2021 (09)
Dr. John Campbell – Pfizer’s pivotal covid-19 vaccine trial, raise questions about data integrity and regulatory oversight (10)
This was the original paper.
Ventavia Research Group (11) – Researchers were testing Pfizer’s vaccine at several sites in Texas. A regional director, Brook Jackson has told The BMJ that the company:
- falsified data
- unblinded patients
- employed inadequately trained vaccinators
- was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial
- Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding.
- US Food and Drug Administration (FDA) were informed
- Ventavia fired her later the same day.
- The BMJ has been provided with dozens of internal company documents, photos, audio recordings, and emails.
- She repeatedly informed her superiors
- poor laboratory management
- patient safety concerns
- data integrity issues
- that drug assignment confirmation printouts were being left in participants’ charts, accessible to blinded personnel (later corrected)
- company wasn’t able to quantify the types and number of errors they were finding when examining the trial paperwork for quality control
- ICON highlighted over 100 outstanding queries older than three days (12)
- Worries over FDA inspection
- Concerns raised
- Participants placed in a hallway after injection and not being monitored by clinical staff
- Lack of timely follow-up of patients who experienced adverse events
- Protocol deviations not being reported
- Vaccines not being stored at proper temperatures
- Mislabelled laboratory specimens
- Targeting of Ventavia staff for reporting these types of problems.
- FDA advisory committee meeting held on 10 December 2020
- Problems at Ventavia not mentioned
- The next day the FDA issued the authorisation of the vaccine
- In August this year, after full FDA approval of Pfizer’s vaccine
- FDA published that 9 of the trials 153 sites were inspected
- FDA, full trial swabs were not taken from 477 people with suspected cases of symptomatic covid-19
Other employees’ accounts
- everything that you complained about was spot on
- Two former Ventavia employees spoke to The BMJ anonymously for fear of reprisal and loss of job prospects in the tightly knit research community
It’s a crazy mess
Pfizer has hired Ventavia as a research subcontractor on four other vaccine clinical trials:
- covid-19 vaccine in children and young adults
- pregnant women
- a booster dose
- an RSV vaccine trial
NCT04816643, NCT04754594, NCT04955626, NCT05035212.
Dr. Suneel Dhand – MedStoic Lifestyle Medicine (13) – BREAKING NEWS in major medical publication. I review this investigative article in the British Medical Journal. What did they say?
Dr Aseem Mulhotra, Consultant Cardiologist – Pfizer Data Scandal (14) – LBC presenter Maajid Nawaz discusses major concerns around vaccine mandates with Consultant Cardiologist Dr Aseem Malhotra who says the corporate capture of public health is at the root of the healthcare crisis.

Site Notifications/Chat:
- Telegram Post Updates @JourneyToABetterLife (channel)
- Telegram Chatroom @JourneyBetterLifeCHAT (say hi / share info)
- Gettr Post Updates @chesaus (like fakebook)
Videos:
References











![[Legal] Dr David Martin – Paper trail as far back as 1999 leads to Current Pandemic](https://pennybutler.com/wp-content/uploads/2022/01/drdavidmartin-777x437.jpg)
![Hands off our kids! [C19 Aussie School Safety Letters]](https://pennybutler.com/wp-content/uploads/2022/02/fairbusinessaustralia-543x437.jpg)
![[Q&A] PANDA 2021 – UGLY Truth About The Covid 19 Lockdowns (Part Two)](https://pennybutler.com/wp-content/uploads/2023/04/FauciScience-2020-AllDeaths-Covid.jpg)