FDA Rapid Covid-19 Tests Recall List
Need this for another post I’m working on.
FDA Recalls of Rapid Antigen Tests
- Do Not Use E25Bio COVID-19 Tests: FDA Safety Communication
- https://www.fda.gov/medical-devices/safety-communications/do-not-use-e25bio-covid-19-tests-fda-safety-communication
- Risk of injury when self-collecting nasopharyngeal or oropharyngeal samples. Only trained health care providers should collect nasopharyngeal or oropharyngeal swab samples. People who were instructed to collect their own nasopharyngeal or oropharyngeal swab samples are at risk of serious injury including:
- Pieces of the swab breaking off, as they may be more fragile and are not designed for throat swabbing, nasopharyngeal swabbing, or self-collection
- The FDA has classified the recall for these tests as a Class I recall, the most serious type of recall.
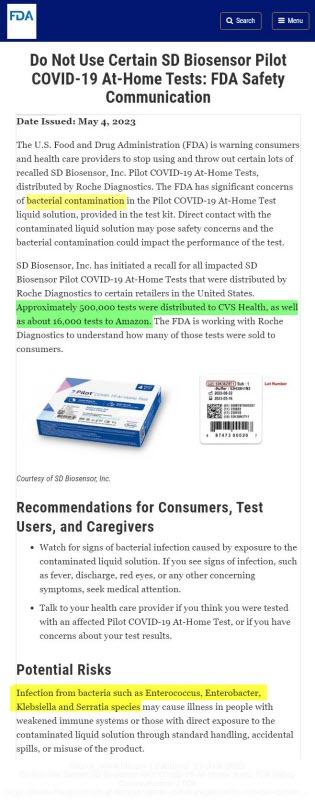
- Do Not Use Certain SD Biosensor Pilot COVID-19 At-Home Tests: FDA Safety Communicationhttps://www.fda.gov/medical-devices/safety-communications/do-not-use-certain-sd-biosensor-pilot-covid-19-home-tests-fda-safety-communication
- The U.S. Food and Drug Administration (FDA) is warning consumers and health care providers to stop using and throw out certain lots of recalled SD Biosensor, Inc. Pilot COVID-19 At-Home Tests, distributed by Roche Diagnostics. The FDA has significant concerns of bacterial contamination in the Pilot COVID-19 At-Home Test liquid solution, provided in the test kit. Direct contact with the contaminated liquid solution may pose safety concerns and the bacterial contamination could impact the performance of the test.
- The liquid solution provided in the affected Pilot COVID-19 At-Home Test kits has been found to be contaminated with organisms such as Enterococcus, Enterobacter, Klebsiella and Serratia species.
- Who: SD Biosensor recalled its Pilot COVID-19 At-Home Tests.
- Why: The company found potentially harmful bacteria inside the tube containing liquid.
- Where: The SD Biosensor recall is active in the United States.
- https://topclassactions.com/lawsuit-settlements/consumer-products/recalls/sd-biosensor-announces-pilot-covid-19-at-home-tests-recall-due-to-bacterial-contamination/
- Potential for False Positive Results with Certain Lots of Ellume COVID-19 Home Tests Due to a Manufacturing Issue: FDA Safety Communication
- The FDA has classified the recall of the Ellume COVID-19 Home Test as a Class I recall, the most serious type of recall. Ellume has identified additional affected lots since our last update on October 5, 2021, and the total of affected tests is now around 2 million.
- https://www.fda.gov/medical-devices/safety-communications/potential-false-positive-results-certain-lots-ellume-covid-19-home-tests-due-manufacturing-issue-fda
- Ellume Recalls COVID-19 Home Test for Potential False Positive SARS-CoV-2 Test Results
- https://www.fda.gov/medical-devices/medical-device-recalls/ellume-recalls-covid-19-home-test-potential-false-positive-sars-cov-2-test-results
- Stop Using Empowered Diagnostics COVID-19 Tests: FDA Safety Communication
- The U.S. Food and Drug Administration (FDA) is warning people to stop using the Empowered Diagnostics CovClear COVID-19 Rapid Antigen Test and ImmunoPass COVID-19 Neutralizing Antibody Rapid Test.
- https://www.fda.gov/medical-devices/safety-communications/stop-using-empowered-diagnostics-covid-19-tests-fda-safety-communication
- The FDA has classified the recall for these tests as a Class I recall, the most serious type of recall.
- Risk of False Results with the Curative SARS-Cov-2 Test for COVID-19: FDA Safety Communication
- Stop Using LuSys Laboratories COVID-19 Tests: FDA Safety Communication
- The FDA has classified the recall for these tests as a Class I recall, the most serious type of recall.
- https://www.fda.gov/medical-devices/safety-communications/stop-using-lusys-laboratories-covid-19-tests-fda-safety-communication
- LuSys Laboratories, Inc Recalls COVID-19 Antigen Tests (Nasal/Saliva) and COVID-19 IgG/IgM Antibody Tests Because They Are Not Authorized, Cleared, or Approved by the FDA
- https://www.fda.gov/medical-devices/medical-device-recalls/lusys-laboratories-inc-recalls-covid-19-antigen-tests-nasalsaliva-and-covid-19-iggigm-antibody-tests
- Do Not Use Certain ACON Flowflex COVID-19 Tests: FDA Safety Communication
- Do Not Use SD Biosensor STANDARD Q COVID-19 Ag Home Tests: FDA Safety Communication
- https://www.fda.gov/medical-devices/safety-communications/do-not-use-sd-biosensor-standard-q-covid-19-ag-home-tests-fda-safety-communication
- SD Biosensor, Inc. has initiated a recall for all unauthorized SD Biosensor STANDARD Q COVID-19 Ag Home Tests that were distributed in the U.S.. The FDA has classified the recall for these tests as a Class I recall, the most serious type of recall.
- Do Not Use Certain Celltrion DiaTrust COVID-19 Tests: FDA Safety Communication
- https://www.fda.gov/medical-devices/safety-communications/do-not-use-certain-celltrion-diatrust-covid-19-tests-fda-safety-communication
- Class 2 Device Recall Celltrion DiaTrust COVID19 Ag Rapid Test
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=191625
- Do Not Use Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test: FDA Safety Communication
….and hundreds more:
FDA Removed the list of names in 2021, but I found what the Rapid Antigen Test recall list was back on June 23, 2021 Archive
| Manufacturer | Test | Status |
|---|---|---|
| Abbexa Ltd | abx294171 – COVID-19 IgG/IgM Rapid Test Kit | Removed – Should Not Be Distributed |
| Abingdon Health Ltd. | AbC-19™ Rapid Test | Removed – Should Not Be Distributed* |
| Absology Co., Ltd. | Absoludy COVID-19 IgM/IgG Combo | Removed – Should Not Be Distributed* |
| Absology Co., Ltd. | Absoludy COVID-19 IgM/IgG RAPID | Removed – Should Not Be Distributed* |
| Absology Co., Ltd. | INIST COVID-19 IgM/IgG RAPID | Removed – Should Not Be Distributed* |
| Accel Diagnostics, LLC | Rapid C2T Total Ab (IgG/IgM) Card | Removed – Should Not Be Distributed* |
| ACCOBiotech SDN.BHD | ACCO COVID-19 IgM/IgG TEST | Removed – Should Not Be Distributed |
| Aegis Sciences Corporation | SARS-CoV-2 Antibody, IgG assay | Removed – Should Not Be Distributed* |
| AESKU.DIAGNOSTICS GmbH & Co. KG | AESKULISA® SARS-CoV-2 NP IgG | Removed – Should Not Be Distributed* |
| AESKU.DIAGNOSTICS GmbH & Co. KG | AESKULISA® SARS-CoV-2 NP IgM | Removed – Should Not Be Distributed* |
| AESKU.DIAGNOSTICS GmbH & Co. KG | AESKULISA® SARS-CoV-2 S1 IgM | Removed – Should Not Be Distributed* |
| AESKU.DIAGNOSTICS GmbH & Co. KG | AESKULISA® SARS-CoV-2 S1 IgG | Removed – Should Not Be Distributed* |
| Agilent Technologies, Inc | Agilent Dako SARS-CoV-2 IgG ELISA kit | Removed – Should Not Be Distributed* |
| Akston Biosciences Corporation | AntiCoV-ID IgG ELISA Test | Removed – Should Not Be Distributed |
| Alfa Scientific Designs, Inc. | Clarity COVID-19 IgG/IgM Antibody Test | Removed – Should Not Be Distributed |
| Alfa Scientific Designs, Inc. | Instant-view plus COVID-19 IgG/IgM Antibody Test | Removed – Should Not Be Distributed |
| ALPCO | SARS-CoV-2 IgG Chemiluminescence ELISA | Removed – Should Not Be Distributed* |
| ALPCO | SARS-CoV-2 IgG ELISA | Removed – Should Not Be Distributed* |
| ALPCO | SARS-CoV-2 IgM Chemiluminescence ELISA | Removed – Should Not Be Distributed* |
| ALPCO | SARS-CoV-2 IgM ELISA | Removed – Should Not Be Distributed* |
| Alpha Pharma Service srl | IRIS SARS-CoV-2 IgG/IgM Rapid Test | Removed – Should Not Be Distributed |
| AngelWorld Biotech Manufacturing FZ LLC | AngelWorld V2.0 COVID-19 IgG/IgM Antibody Rapid Test | Removed – Should Not Be Distributed |
| Anhui Deepblue Medical Technology Co., Ltd. | COVID-19 (SARS-CoV-2) IgG/IgM Antibody Test Kit (Colloidal Gold) | Removed – Should Not Be Distributed |
| Ansh Labs | SARS-CoV2 IgG ELISA (AL-1001) | Removed – Should Not Be Distributed |
| Antagen Pharmaceuticals, Inc. | COVID-19 IgM/IgG Antibody Test Kit (Colloidal Gold) | Removed – Should Not Be Distributed* |
| Antibody BioPharm, Inc. | COVID-19 IgM/IgG POCT Kit (Colloidal Gold) | Removed – Should Not Be Distributed* |
| AnyGo Technology Co., Ltd | COVID-19 Capture ELISA Assay | Removed – Should Not Be Distributed* |
| Arbor Assays | DetectX SARS-CoV-2 IgG ELISA Kit | Removed – Should Not Be Distributed* |
| Arbor Vita Corporation | CoVisa IgG Test | Removed – Should Not Be Distributed |
| Artron BioResearch Inc./ Artron Laboratories Inc. | COVID-19 IgM/IgG Antibody Test | Removed – Should Not Be Distributed* |
| Asan Pharmaceutical Co., Ltd. | Asan Easy Test COVID-19 IgG/IgM | Removed – Should Not Be Distributed* |
| Atlas Link (Beijing)Technology Co., Ltd | NovaTest: One Step COVID-19 IgG/IgM rapid test | Removed – Should Not Be Distributed |
| Audacia Bioscience | CMC-19D SARS-CoV2 (COVID-19) Rapid Antibody Test | Removed – Should Not Be Distributed |
| Aurora Biomed Inc. | COVID-19 IgG/IgM Rapid Test Cassette (Colloidal Gold) | Removed – Should Not Be Distributed |
| Autobio Diagnostics Co. Ltd. | Anti-SARS-CoV-2 Rapid Test | Removed – Should Not Be Distributed |
| Axium BioResearch, Inc. | AxiumHealth COVID-19 IgG/IgM Antibody Test | Removed – Should Not Be Distributed* |
| Beacon Analytical Systems, Inc. | SARS-CoV-2 Human IgG Antibody Test 96-Well Microtiter Plate Kit Format | Removed – Should Not Be Distributed* |
| Beijing Beier Bioengineering Co., Ltd | 2019-New Coronavirus IgM/IgG Rapid Test Cassette (WB/S/P) | Removed – Should Not Be Distributed |
| Beijing Decombio Biotechnology Co., Ltd. | Novel Coronavirus IgM/IgG Combo Rapid Test-Cassette (Serum/Plasma/Whole blood) | Removed – Should Not Be Distributed* |
| Beijing DiaGreat Biotechnologies Co. Ltd. | 2019-nCoV IgM Antibody Determination Kit | Removed – Should Not Be Distributed* |
| Beijing DiaGreat Biotechnologies Co. Ltd. | 2019-nCoV IgG/IgM Antibody Rapid Test Kit | Removed – Should Not Be Distributed* |
| Beijing DiaGreat Biotechnologies Co. Ltd. | 2019-nCoV IgG Antibody Determination Kit | Removed – Should Not Be Distributed* |
| Beijing Kewei Clinical Diagnostic Reagent Inc. | Genonto RapidTest10 COVID-19 IgG/IgM Antibody Rapid Test Kit | Removed – Should Not Be Distributed |
| Beijing O&D BIOTECH Co., LTD. | Coronavirus disease (COVID-19) Total Antibody Rapid Test (Colloidal Gold) | Removed – Should Not Be Distributed |
| Beroni Group | SARS-CoV-2 IgG/IgM Antibody Detection Kit | Removed – Should Not Be Distributed |
| BestNovo (Jiangsu) Medical Technology Co., Ltd. | BestNovo COVID-19 IgM/IgG Antibody Rapid Test Kit | Removed – Should Not Be Distributed |
| Bethyl Laboratories, Inc. | Bethyl SARS-CoV-2 IgG IVD ELISA | Removed – Should Not Be Distributed* |
| Biobase Biodustry (Shandong) Co., Ltd. | SARS-CoV-2 IgM/IgG Antibody Test Kit (Colloidal Gold) | Removed – Should Not Be Distributed |
| Biocan Diagnostics Inc. | Tell Me Fast Novel Coronavirus (COVID-19) IgG/IgM Antibody Test (Strip Format) | Removed – Should Not Be Distributed* |
| Biolidics Limited | 2019-nCoV IgG/IgM Detection Kit (Colloidal Gold) | Removed – Should Not Be Distributed* |
| BioMedomics, Inc. | COVID-19 IgM-IgG rapid test | Removed – Should Not Be Distributed* |
| Biomerica, Inc. | COVID-19 IgG/IgM Rapid Test | Removed – Should Not Be Distributed |
| Bioscience Diagnostic Technology Company Ltd. | Diagnostic Kit for Novel Coronavirus (2019-nCoV) IgG Antibody | Removed – Should Not Be Distributed |
| Bioscience Diagnostic Technology Company Ltd. | Diagnostic Kit for Novel Coronavirus (2019-nCoV) IgM Antibody | Removed – Should Not Be Distributed |
| Bioscience(Chongqing) Diagnostic Technology Co., Ltd. | Qualitative Diagnostic Kit for Novel Coronavirus (2019-nCoV) IgM Antibody | Removed – Should Not Be Distributed |
| Bioscience(Chongqing) Diagnostic Technology Co., Ltd. | Qualitative Diagnostic Kit for Novel Coronavirus (2019-nCoV) IgG Antibody | Removed – Should Not Be Distributed |
| Bioscience(Tianjin) Diagnostic Technology Co., Ltd. | Qualitative Diagnostic Kit for Novel Coronavirus(2019-nCoV) IgM Antibody | Removed – Should Not Be Distributed |
| Bioscience(Tianjin) Diagnostic Technology Co., Ltd. | Qualitative Diagnostic Kit for Novel Coronavirus(2019-nCoV) IgG Antibody | Removed – Should Not Be Distributed |
| Bioscience(Tianjin) Diagnostic Technology Co., Ltd. | Novel Coronavirus (2019-nCoV) Antibody Rapid Test | Removed – Should Not Be Distributed |
| BioSure (UK) Limited | BioSURE COVID-19 Antibody Rapid Test (REF: 92001COV) | Removed – Should Not Be Distributed* |
| BioSys Laboratories, Inc. | BioSys Plus COVID-19 IgM/IgG Rapid Test | Removed – Should Not Be Distributed |
| Bloom Diagnostics AG | Bloom COVID-19 Test | Removed – Should Not Be Distributed* |
| Boston Bio Lab Inc. | Boston Bio EZ Covid-19 | Removed – Should Not Be Distributed* |
| Boston Biopharma Inc. | BB Rapid-MT | Removed – Should Not Be Distributed* |
| BreviTest Technologies, LLC | Brevitest SARS-CoV-2 IgG Test | Removed – Should Not Be Distributed* |
| BTNX, Inc. | Rapid Response™ COVID-19 IgG/IgM Test Cassette | Removed – Should Not Be Distributed* |
| Calbiotech, Inc. | ErbaLisa® COVID-19 IgG | Removed – Should Not Be Distributed |
| Camtech Diagnostics Pte Ltd | Camtech Covid-19 IgM/IgG 2C Rapid Test | Removed – Should Not Be Distributed |
| Camtech Diagnostics Pte Ltd | Camtech Covid-19 IgM/IgG 1C Rapid Test | Removed – Should Not Be Distributed |
| Cell ID Pte Ltd | Smart COVID-19 IgM/IgG Rapid Diagnostic Test (RDT) | Removed – Should Not Be Distributed* |
| Changchun Wancheng Bio-Electron Co., Ltd. | COVID-19 IgG/IgM ANTIBODY RAPID TEST KIT (Colloidal gold immunochromatography) | Removed – Should Not Be Distributed |
| Chembio Diagnostic Systems, Inc. | DPP COVID-19 IgM/IgG System | Removed – Should Not Be Distributed |
| Chemtron Biotech, Inc. | Chemtrue®Rapid COVID-19 IgM/IgG Antibody Test | Removed – Should Not Be Distributed |
| ClearSign Diagnostics Co | Novel Coronavirus (SARS-CoV-2) Antibody (IgM / IgG) Test | Removed – Should Not Be Distributed |
| ClearSign Diagnostics Co., LLC | ClearSign Diagnostics SARS-CoV-2 Antibody (IgM and IgG) Test | Removed – Should Not Be Distributed |
| Core Technology Co., Ltd. | RapidTest COVID-19 IgM/IgG Ab Test | Removed – Should Not Be Distributed |
| CoreMedica Laboratories, Inc. | Capillary Blood Collection Kit & Laboratory Analysis for SARS-CoV-2 ELISA IgG and IgM | Removed – Should Not Be Distributed |
| CTK Biotech, Inc. | OnSite® COVID-19 IgG/IgM Rapid Test | Removed – Should Not Be Distributed |
| Diabetomics, Inc | CovAbScreen™ SARS-CoV-2 Ab Test | Removed – Should Not Be Distributed* |
| DIALAB(ZJG) Biotech Co., Ltd. | SARS-CoV-2 IgG/IgM Antibody Test (Fluorescence Immunoassay) | Removed – Should Not Be Distributed* |
| Diazyme Laboratories, Inc. | Diazyme SARS-CoV-2 Antibody Rapid Test | Removed – Should Not Be Distributed* |
| Discount Diagnostics | Global WholeHealth Partners RDT IgG/IgM Antibody test | Removed – Should Not Be Distributed |
| DLS Research & Ventures | SCOV Immune Response Test | Removed – Should Not Be Distributed |
| DLS Research & Ventures | Screen For Coronaviruses (SCOV) Immune Response Test | Removed – Should Not Be Distributed |
| DLS Research & Ventures | Screen For Coronaviruses (SCOV) Test | Removed – Should Not Be Distributed |
| DNALink, Inc. | AccuFind COVID19 IgG | Removed – Should Not Be Distributed* |
| DxGen Corp. | Epithod616 COVID-19 IgG Test Kit | Removed – Should Not Be Distributed* |
| DxGen Corp. | EDGC RapidCheck COVID-19 IgG Test Kit | Removed – Should Not Be Distributed* |
| Dynamiker Biotechnology (Tianjin) Co., Ltd. | RapidCOV™ 2019-nCOV IgG/IgM Rapid Test | Removed – Should Not Be Distributed |
| EACHY Biopharmaceuticals Co., Ltd. | AccuRapid™ SARS-CoV-2 IgM/IgG Test Kit (Fluorescence Lateral Flow Immunoassay) | Removed – Should Not Be Distributed |
| EACHY Biopharmaceuticals Co., Ltd. | AccuRapid™ SARS-CoV-2 IgM/IgG Test Kit (Lateral Flow Immunoassay) | Removed – Should Not Be Distributed |
| EACHY Biopharmaceuticals Co., Ltd. | SmartScreen COVID-19 IgM/IgG Test Kit | Removed – Should Not Be Distributed |
| Edinburgh Genetics Limited | ActivXpress+ COVID-19 IgG/IgM Immunoassay Complete Testing Kit | Removed – Should Not Be Distributed* |
| EpiGentek | SeroFlash SARS-CoV-2 IgM/IgG Antibody Detection Kit | Removed – Should Not Be Distributed* |
| Epitope Diagnostics, Inc. | EDI COVID-19 Nucleocapsid IgG Quantitative ELISA Kit | Removed – Should Not Be Distributed |
| Epitope Diagnostics, Inc. | KT-1033 EDI™ Novel Coronavirus COVID-19 IgG ELISA Kit | Removed – Should Not Be Distributed* |
| Epitope Diagnostics, Inc. | KT-1032 EDI™ Novel Coronavirus COVID-19 IgG ELISA Kit | Removed – Should Not Be Distributed* |
| ET Healthcare Inc. | Pylon COVID-19 IgM/IgG Assay | Removed – Should Not Be Distributed* |
| EUROIMMUN AG | Anti-SARS-CoV-2 ELISA (IgA) | Removed – Should Not Be Distributed* |
| Fosun Pharma USA Inc. | Fosun COVID-19 IgG/IgM Rapid Antibody Detection Kit | Removed – Should Not Be Distributed* |
| Genabio LLC | SARS-CoV-2 IgM/IgG (GICA) Test | Removed – Should Not Be Distributed |
| GenBody Inc. | GenBody COVID-19 IgM/IgG | Removed – Should Not Be Distributed |
| Genelogic Research Limited | Genelogic COVID-19 IgM/IgG Antibody Rapid Test | Removed – Should Not Be Distributed |
| General Biologicals Corporation | GB SARS-CoV-2 Ab ELISA, Cat. No.:4ECO001 | Removed – Should Not Be Distributed* |
| GeneScan Diagnostics, LLC | AmeriDx® SARS-CoV-2 IgG/IgM Rapid Test Kit | Removed – Should Not Be Distributed |
| GeneScan Diagnostics, LLC | AmeriDx® SARS-CoV-2 IgG/IgM-T Antibody Rapid Test Kit | Removed – Should Not Be Distributed |
| Genesprint Group, Ltd. | Genesprint Diagnostic Kit for IgG/IgM Antibody to SARS-CoV-2 test | Removed – Should Not Be Distributed* |
| Genlantis Diagnostics, Inc. | CovidQuik Coronavirus (COVID-19) IgM/IgG Antibody Test | Removed – Should Not Be Distributed* |
| Genobio Pharmaceutical Co., Ltd. | COVID-19 IgM/IgG Lateral Flow Assay | Removed – Should Not Be Distributed* |
| Genrui Biotech Inc. | Novel Coronavirus (2019-nCoV) IgG/IgM Test Kit (Colloidal Gold) | Removed – Should Not Be Distributed |
| Getein Biotech Inc. | One Step Test for Novel Coronavirus (2019-nCoV) IgM/IgG antibody (Colloidal Gold) | Removed – Should Not Be Distributed |
| Global Go (Distributed Core Technology Co., Ltd.) | RapidTest COVID-19 IgM/IgG Ab Test | Removed – Should Not Be Distributed |
| Gold Standard Diagnostics | SARS-CoV-2 IgM ELISA Test Kit | Removed – Should Not Be Distributed |
| Gold Standard Diagnostics | INgezim® COVID 19 CROM Rapid Test Kit | Removed – Should Not Be Distributed* |
| Gold Standard Diagnostics | INgezim® COVID 19 DR ELISA Test Kit | Removed – Should Not Be Distributed* |
| Goldsite Diagnostics Inc | SARS-CoV-2 IgG/IgM kit | Removed – Should Not Be Distributed* |
| GREEN ENERGY TECHNOLOGY SOLUTIONS, LLC | AMP Rapid Test SARS-CoV-2 IgG/IgM | Removed – Should Not Be Distributed* |
| Guangdong Hecin Scientific, Inc. | SARS-CoV-2 IgM Antibody Rapid Test Kit | Removed – Should Not Be Distributed* |
| Guangzhou Decheng biotechnology | DOCHEK™ SARS-CoV-2 Antibody Rapid Test Kit | Removed – Should Not Be Distributed* |
| Guangzhou Fenghua Bioengineering Co., Ltd. | SARS-CoV-2 IgG/IgM Rapid Test | Removed – Should Not Be Distributed |
| H&Z Life Science Co. Ltd. | Anti-COVID-19 Virus IgM/IgG Test Kit (lateral flow assay) | Removed – Should Not Be Distributed |
| H-Guard (China) Co., Ltd. | Novel Coronavirus COVID-19 IgM/IgG Test Kit (colloidal gold) | Removed – Should Not Be Distributed* |
| Hangzhou AllTest Biotech Co., Ltd. | AllTest 2019-nCoV IgG/IgM Rapid Test Cassette | Removed – Should Not Be Distributed |
| Hangzhou AllTest Biotech Co., Ltd. | AllTest COVID-19 IgG/IgM Rapid Test Strip | Removed – Should Not Be Distributed |
| Hangzhou Clongene Biotech Co., Ltd. | COMBRA COVID-19 IgM/IgG Rapid Test Cassette | Removed – Should Not Be Distributed |
| Hangzhou Realy Tech Co Ltd. | 2019-nCOV IgG/IgM Rapid Test | Removed – Should Not Be Distributed |
| Hangzhou Sejoy Electronics & Instrument Co., Ltd. | COVID-19 IgG/IgM Rapid Test Cassette | Removed – Should Not Be Distributed* |
| Hangzhou Testsea Biotechnology Co., Ltd. | One Step SARS-CoV2(COVID-19) IgG/IgM Test | Removed – Should Not Be Distributed* |
| Horizon Pharmaceuticals, Inc. | Horizon RDT SARS CoV-2 IgM/IgG Antibody Test | Removed – Should Not Be Distributed |
| Hunan RunKun Pharmaceutical Co., Ltd. | SARS-CoV-2 lgM/lgG Test Kit (Colloidal Gold) | Removed – Should Not Be Distributed* |
| ICT international | 2019-nCoV IgG/IgM Rapid Test Cassette | Removed – Should Not Be Distributed* |
| IMMY, Inc. | clarus SARS-CoV-2 Total Antibody EIA | Removed – Should Not Be Distributed* |
| Innovation Biotech (Beijing) Co., Ltd. | SARS-COV-2 IgM/IgG Antibody Rapid Test (Immunochromatographic Method) | Removed – Should Not Be Distributed |
| Invenio Medical | COVID-19 IgG/IgM Rapid Test Cassette (WB/S/P) | Removed – Should Not Be Distributed |
| Invenio Medical | COVID-19 IgG/IgM Ab Rapid Test | Removed – Should Not Be Distributed |
| Iontox | COVID-19 (SARS-CoV-2) IgG antibody test kit | Removed – Should Not Be Distributed* |
| Jiangsu Dablood Pharmaceutical Co, Ltd. | AssuranceAB™ COVID-19 IgM/IgG Rapid Antibody Test | Removed – Should Not Be Distributed* |
| Jiangsu Dablood Pharmaceutical Co. Ltd. | COVID-19 IgM/IgG Rapid Test | Removed – Should Not Be Distributed |
| Jiangsu Eubo Biotechnology Co., Ltd. | EUBO COVID-19 IgG/IgM Rapid Test Cassette (WB/S/P) | Removed – Should Not Be Distributed |
| Jiangsu Macro & Micro-Test Med-Tech Co., Ltd. | SARS-CoV-2 IgM/IgG Rapid Assay Kit (Colloidal Gold) | Removed – Should Not Be Distributed* |
| Jiangsu Superbio Biomedical (Nanjing) Co., Ltd. | SARS-CoV-2 (COVID-19) IgM/IgG Antibody Fast Detection Kit (Colloidal Gold) | Removed – Should Not Be Distributed* |
| Jiangsu Superbio Biomedical (Nanjing) Co., Ltd. | ThermoGenesis SARS-CoV-2(COVID-19) IgM/IgG Antibody Fast Detection Kit (Colloidal Gold) | Removed – Should Not Be Distributed* |
| JOYSBIO (Tianjin) Biotechnology Co., Ltd. | COVID-19 IgG/IgM Rapid Test Kit (Colloidal Gold) | Removed – Should Not Be Distributed |
| Leinco Technologies, Inc. | COVID-19 ImmunoRank Neutralization MICRO-ELISA | Removed – Should Not Be Distributed |
| Lepu Medical Technology (Beijing) Co., Ltd. | Lepu SARS-CoV-2 Antibody Rapid Test | Removed – Should Not Be Distributed |
| Leritas LLC | DualTec SARS-CoV-2 Test Kit | Removed – Should Not Be Distributed* |
| Lifeassay Diagnostics (Pty) Ltd | Test-it COVID-19 IgM/IgG Lateral Flow Assay | Removed – Should Not Be Distributed |
| LumiQuick Diagnostics, Inc. | QuickProfile™ 2019-nCoV IgG/IgM Antibody Test | Removed – Should Not Be Distributed |
| Maccura Biotechnology Co., Ltd. | Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) IgM/IgG Antibody Assay Kit by Colloidal Gold Method | Removed – Should Not Be Distributed* |
| MedicalSystem Biotechnology Co., Ltd. | Coronavirus Disease 2019 Antibody (IgM/IgG) Combined Test Kit | Removed – Should Not Be Distributed* |
| Medicon Company Limited | Trueline COVID-19 IgG/IgM Rapid Test | Removed – Should Not Be Distributed |
| MedMira Inc. | REVEALCOVID-19™ Total Antibody Test | Removed – Should Not Be Distributed* |
| Melbourne Biotech | MB COVID-19 Antibody Detection Kit | Removed – Should Not Be Distributed |
| Mokobio Biotechnology R&D Center | SARS-CoV-2 IgM & IgG Quantum Dot Immunoassay | Removed – Should Not Be Distributed* |
| Mokobio Biotechnology R&D Center Inc | COVID-19 IgM & IgG Rapid Test (Colloidal Gold Assay) | Removed – Should Not Be Distributed* |
| Molecular Innovations | SARS-CoV-2 IgG Seroconversion ELISA Kit | Removed – Should Not Be Distributed* |
| Multi-G bvba | COVID-19 IgG/IgM Rapid Test Cassette | Removed – Should Not Be Distributed* |
| Nanjing Norman Biological Technology Co., Ltd | Novel Coronavirus (2019-nCoV) IgG/IgM Antibody Testing Kit (Colloidal Gold) | Removed – Should Not Be Distributed* |
| Nanjing SYNTHGENE Medical Technology Co., Ltd. | SYNTHGENE COVID-19 IgM/IgG Rapid Detection Kit (Colloidal Gold Method) | Removed – Should Not Be Distributed |
| Nanjing Vazyme Medical Technology Co., LTD. | 2019-nCoV IgG / IgM Detection Kit (Colloidal Gold-Based) | Removed – Should Not Be Distributed* |
| Nanjing Weiyun Biotechnology Co., Ltd. | SARS-CoV-2 (COVID-19) IgM/IgG Antibody Fast Detection Kit (Colloidal Gold) | Removed – Should Not Be Distributed |
| Nano-Ditech Corporation | Nano-Check COVID-19 IgG/IgM Antibody Test | Removed – Should Not Be Distributed* |
| NanoEntek, Inc. | FREND COVID-19 IgG/IgM Duo | Removed – Should Not Be Distributed* |
| Nanomix, Inc. | Nanomix eLab® COVID-19 Rapid IgG/IgM Panel | Removed – Should Not Be Distributed* |
| NanoResearch, Inc. | NanoMedicina™ SARS-COV-2 IgM/IgG Antibody Rapid Test | Removed – Should Not Be Distributed |
| Nantong Diagnos Biotechnology Co., Ltd. | (2019-nCoV) New coronavirus Antibody Test (Colloidal Gold) | Removed – Should Not Be Distributed |
| Nantong Egens Biotechnology Co., Ltd | EGENS COVID-19 IgG/IgM Rapid Test Kit | Removed – Should Not Be Distributed |
| Naturitious LLC | Viralert COVID-19 IgG/IgM ANTIBODY RAPID TEST KIT | Removed – Should Not Be Distributed* |
| Newscen Bio-Pharmaceutical Co., Ltd. | COVID-19 Microfluidic IgG/IgM Test | Removed – Should Not Be Distributed |
| Newscen Coast Bio-Pharmaceutical Co., Ltd. | COVID-19 Microfluidic Combo Test | Removed – Should Not Be Distributed |
| Newscen Coast Bio-Pharmaceutical Co., Ltd. | COVID-19 IgG/IgM Rapid Test | Removed – Should Not Be Distributed |
| Oncosem Onkolojik Sistemler San.ve Tic. A.S | CRT (COVID-19 Rapid Test) | Removed – Should Not Be Distributed* |
| One Lambda, Inc. | LABScreen™ COVID Plus | Removed – Should Not Be Distributed* |
| One Milo, Inc. | InstantRapid® SARS CoV-2 IgG IgM Test | Removed – Should Not Be Distributed |
| Otogenetics | OTO-Artron COVID-19 IgM/IgG Antibody Rapid Detection test | Removed – Should Not Be Distributed* |
| Pacific Connect Group LLC (US) | SARS-CoV-2 IgG/IgM Rapid Test Kit | Removed – Should Not Be Distributed |
| PCL Inc. | COVID19 IgG/IgM Rapid Gold | Removed – Should Not Be Distributed |
| Pellecome LLC (Distributed Core Technology Co., Ltd.) | CoreTest COVID-19 IgM/IgG Ab Test | Removed – Should Not Be Distributed |
| PerGrande BioTech Development Co., Ltd. | SARS-CoV-2 Antibody Detection Kit (Colloidal Gold Immunochromatographic assay) | Removed – Should Not Be Distributed* |
| Phamatech | COVID19 IgG / IgM Rapid Test | Removed – Should Not Be Distributed* |
| Pharmact GmbH | BelTEST It SARS CoV-2 IgM/IgG Rapid Test (Pharmact CoV-2 Rapid Test) | Removed – Should Not Be Distributed* |
| Pinnacle Biolabs | SARS-CoV-2 IgG/IgM Rapid Test | Removed – Should Not Be Distributed |
| Plexense, Inc. | ACCEL ELISA COVID-19 | Removed – Should Not Be Distributed* |
| Profarma, UAB | ProRapid Coronavirus Disease (SARS-CoV-2) IgG/IgM Rapid Test Cassette Single Use Kit | Removed – Should Not Be Distributed |
| ProGnosis Biotech SA | Bio-Shield 2019-nCoV Total Ig ELISA | Removed – Should Not Be Distributed* |
| Promedical | COVID-19 Rapid Test | Removed – Should Not Be Distributed |
| Promedical Equipment Pty Ltd | Promedical IgM/IgG Rapid Detection Kit | Removed – Should Not Be Distributed |
| Prometheus Bio Inc. | 2019-nCoV IgG/IgM Test (Whole blood/Serum/Plasma) Colloidal Gold | Removed – Should Not Be Distributed* |
| ProteomeTech Inc. | KOVIcheck COVID-19 IgG/IgM | Removed – Should Not Be Distributed* |
| Pure Genetic Medical Ltd. (Distributed Boson Biotech Ltd. Co) | Rapid 2019-nCoV IgG/IgM Combo Test Card | Removed – Should Not Be Distributed |
| Qualigen, Inc. | FastPack® SARS-CoV-2 IgG Immunoassay | Removed – Should Not Be Distributed* |
| RayBiotech Life, Inc. | Novel Coronavirus (SARS-CoV-2) IgM and IgG Antibody Detection Kits for Serum | Removed – Should Not Be Distributed |
| RayBiotech Life, Inc. | Novel Coronavirus (SARS-CoV-2) IgM and IgG Dual Combined Antibody Detection Kit for Serum | Removed – Should Not Be Distributed |
| Reszon Diagnostics International Sdn. Bhd. | RESZON (COVID-19) (Sars-Cov-2) IgG/IgM Antibody Test | Removed – Should Not Be Distributed* |
| Reszon Diagnostics International Sdn. Bhd. | Reszon COVID-19 Rapid IgG/IgM Test | Removed – Should Not Be Distributed* |
| Ring Biotechnology Co Ltd | COVID-19 IgM/IgG Rapid Test Kit | Removed – Should Not Be Distributed* |
| SA Scientific | SAS™ COVID-19 Antibody Detection test | Removed – Should Not Be Distributed* |
| Safecare Biotech (Hagzhou) Co., Ltd. | SAFECARE COVID-19 IgG/IgM Rapid Test Device | Removed – Should Not Be Distributed |
| Saladax Biomedical | COVID-19 IgG/IgM Rapid Antibody Test | Removed – Should Not Be Distributed* |
| ScheBo® • Biotech AG ScheBo® | SARS-CoV-2 Quick | Removed – Should Not Be Distributed* |
| Sciteck Diagnostics | COVID19 Automated Antibody Assay | Removed – Should Not Be Distributed |
| SD Biosensor | STANDARD Q COVID-19 IgM/IgG Duo | Removed – Should Not Be Distributed |
| Sensing Self Pte. Ltd. | COVID-19 Rapid IgM/IgG Combined Antibody Assay Pre-Screening Kit | Removed – Should Not Be Distributed |
| Shandong ThinkLab Biotechnology Co., Ltd. | 2019-nCoV IgM/IgG antibody Test Kit (Colloidal-gold Assay) | Removed – Should Not Be Distributed |
| Shanghai Eugene Biotech Co., Ltd. | SARS-CoV2 (COVID-19) IgG/IgM Rapid Test | Removed – Should Not Be Distributed |
| Shanghai Fosun Long March Medical Science Co., Ltd. | Fosun COVID-19 IgG/IgM Rapid Antibody Detection Kit | Removed – Should Not Be Distributed* |
| Shanghai Liangrun Biomedicine Technology Co., Ltd. | Liangrun COVID-19 IgM/IgG Antibody Test | Removed – Should Not Be Distributed |
| Shanghai Outdo Biotech Co., Ltd. | Novel Coronavirus (SARS-CoV-2) Antibody (IgM / IgG) Test | Removed – Should Not Be Distributed* |
| Shenzen Landwind Medical Co., Ltd. | COVID-19 IgG/IgM Rapid Test | Removed – Should Not Be Distributed* |
| Shenzhen Kingfocus Biomedical Engineering Co., Ltd. | True-Dot™ Novel Coronavirus (2019-nCoV) IgM/IgG Rapid Antibody Detection Kit (Quantum Dot Immunochromatography) | Removed – Should Not Be Distributed* |
| Shenzhen Lvshiyuan Biotechnology Co., Ltd | COVID-19 (2019-nCoV) Coronavirus IgG/IgM Rapid Test Kit | Removed – Should Not Be Distributed |
| Shijiazhuang Hipro Biotechnology Co., Ltd. | COVID-19 IgM/IgG Antibody Test Kit (Colloidal Gold) | Removed – Should Not Be Distributed |
| Singuway Biotech Inc. | COVID-19 IgG/IgM Detection Kit (Colloidal Gold) | Removed – Should Not Be Distributed |
| Sinocare, Inc. | SARS-CoV-2 Antibody Test Kit (Colloidal Gold Method) | Removed – Should Not Be Distributed |
| Sober Holdings, LLC | ProMed Rapid Testing – COVID-19 IgG/IgM Test Kit | Removed – Should Not Be Distributed |
| Sober Holdings, LLC | ProMed Rapid Test | Removed – Should Not Be Distributed |
| South Jersey Diagnostics, LLC | COVID-19-CHECK-1 | Removed – Should Not Be Distributed* |
| Spartacus-Biomed | LFA COVID-19 IgG & IgM Rapid Test Device | Removed – Should Not Be Distributed |
| Spring Health Care AG | COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) | Removed – Should Not Be Distributed |
| Sugentech, Inc. | SGTi-flex COVID-19 IgM/IgG | Removed – Should Not Be Distributed* |
| Sunbeam Laboratories, LLC. | Sunbeam Laboratories Covid-19 Rapid Test Kit | Removed – Should Not Be Distributed* |
| Sure Bio-tech | API Covid-Rapid IgM/IgG Antibody Test Kit | Removed – Should Not Be Distributed |
| Sure Bio-Tech | API Pharma Covid-Rapid IgM/IgG Antibody Test | Removed – Should Not Be Distributed |
| SUREDX | V-CHEK™ SARS-CoV-2 Antibody Rapid Test Kit | Removed – Should Not Be Distributed |
| Suzhou Kangheshun Medical Technology Co., Ltd | SARS-CoV-2 IgG/IgM Rapid Test Cassette | Removed – Should Not Be Distributed |
| Telepoint Medical Services | SARS-CoV-2 IgG/IgM Rapid Qualitative Test | Removed – Should Not Be Distributed |
| TheraTest Laboratories, Inc. | EL-Anti-SARS-CoV-2 IgG | Removed – Should Not Be Distributed* |
| Tianjin Beroni Biotechnology Co. Ltd | SARS-CoV-2 IgG/IgM Antibody Detection Kit | Removed – Should Not Be Distributed |
| Tianjin New Bay Bioresearch Co., Ltd. | Quikpac II COVID-19 IgG/IgM | Removed – Should Not Be Distributed* |
| TM Testing, Inc. | TM Test Kits, CoVID-19 Rapid Antibody Serology Test | Removed – Should Not Be Distributed |
| Top Biotech Sdn. Bhd | TOP RAPID COVID-19 Rapid Antibody IgG/IgM Test Kit | Removed – Should Not Be Distributed |
| Truvian Sciences Inc. | Easy Check COVID-19 IgM/IgG™ POC Antibody Test | Removed – Should Not Be Distributed* |
| Universal Meditech Inc. | DiagnosUS® SARS-CoV-2 Antibody (IgG/IgM) Test | Removed – Should Not Be Distributed |
| Velox Biosystems | COVID-19 Microarray IgG Immunoassay | Removed – Should Not Be Distributed* |
| VicTorch Meditek Inc. | COVID-19 Serology IgM ELISA | Removed – Should Not Be Distributed* |
| VicTorch Meditek Inc. | COVID-19 Serology IgG ELISA | Removed – Should Not Be Distributed* |
| VicTorch Meditek Inc. | COVID-19 IgG/IgM Rapid test Cassette | Removed – Should Not Be Distributed* |
| Victory Square Health Canada, Inc. | Safetest-COVID19 IgG / IgM – Elisa Kit | Removed – Should Not Be Distributed* |
| Victory Square Health, Inc. | Safetest Covid-19 IgG/IgM Rapid Test | Removed – Should Not Be Distributed* |
| VirIntel, LLC | VirIntel COVID-19 Antibody Test | Removed – Should Not Be Distributed |
| Virotech Diagnostics GmbH | Gold Standard Diagnostics SARS-CoV-2 IgA ELISA Test Kit | Removed – Should Not Be Distributed* |
| VITA Testing | COVID-19 IgM/IgG Antibody Rapid Test Kit | Removed – Should Not Be Distributed |
| Vitro Diagnostics | Vitro Diagnostics COVID 19 IgG/IgM Rapid Test Cassette Serology Device | Removed – Should Not Be Distributed |
| VivaChek Biotech (Hangzhou) Co., Ltd. | VivaDiag COVID-19 IgM/IgG Rapid Test | Removed – Should Not Be Distributed |
| Vivera Pharmaceuticals, Inc. | Vivera Pharma COVID-19 Rapid Test | Removed – Should Not Be Distributed* |
| Vivera Pharmaceuticals, Inc. | COVx-RT | Removed – Should Not Be Distributed |
| W.H.P.M., Inc. | COVID-19 IgM/IgG Rapid Test | Removed – Should Not Be Distributed |
| W.H.P.M., Inc. | COVISURE™ COVID-19 IgM/IgG Rapid Test | Removed – Should Not Be Distributed |
| Wuhan EasyDiagnosis | Ediagnosis COVID-19 (SARS-CoV-2) IgM/IgG Antibody Test | Removed – Should Not Be Distributed |
| Wuhan UNscience Biotechnology Co., Ltd. | Coronavirus-19 (COVID-19) Antibody (IgM/IgG) Rapid Test Kit (Colloidal gold immunochromatography) | Removed – Should Not Be Distributed* |
| Wuhu 3H Biotechnology Co. Ltd. | COVID-19 IgG/IgM Test Kit (Colloidal Gold Method) | Removed – Should Not Be Distributed |
| Xiamen AmonMed Biotechnology Co., Ltd. | Helix-19 COVID-19 IgM/IgG Test Kit (Colloidal Gold) | Removed – Should Not Be Distributed |
| Xiamen AmonMed Biotechnology Co., Ltd. | COVID-19 IgM/IgG test kit (Colloidal Gold) | Removed – Should Not Be Distributed* |
| Xiamen Wiz Biotech Co., Ltd. | Diagnostic Kit (Colloidal Gold) for IgG/IgM Antibody to SARS-CoV-2 | Removed – Should Not Be Distributed |
| Zalgen Labs, LLC. | ReSARS CoV-2 (N) IgG ELISA | Removed – Should Not Be Distributed* |
| Zepto Life Technology | ZepMIA20 SARS-CoV-2 IgG assay | Removed – Should Not Be Distributed* |
| ZEUS Scientific, Inc. | ZEUS Rapid SARS-CoV-2 IgM/IgG Test System | Removed – Should Not Be Distributed* |
| Zhejiang Orient Gene Biotech, Co., Ltd. | COVID-19 IgG/IgM Rapid Test Cassette | Removed – Should Not Be Distributed |
| Zhengzhou Fortune Bioscience Co., Ltd. | COVID-19 Antibody Rapid Test Kit (Colloidal Gold Immunochromatography Method) | Removed – Should Not Be Distributed |
| Zhengzhou Fortune Bioscience Co., Ltd. | COVID-19 IgM Antibody Rapid Test Kit | Removed – Should Not Be Distributed |
| Zhengzhou Fortune Bioscience Co., Ltd. | COVID-19 IgG Antibody Rapid Test Kit | Removed – Should Not Be Distributed |
| Zhongshan Bio-Tech Co Ltd. | SARS-CoV-2 IgM/IgG (GICA) | Removed – Should Not Be Distributed* |
| Zhuhai Encode Medical Engineering Co., Ltd | Novel Coronavirus (COVID-19) IgG/IgM Rapid Test Device | Removed – Should Not Be Distributed* |
| Zhuhai Keyu Biological Engineering Co., Ltd. | SARS-CoV-2 IgG/IgM Rapid Test Kit | Removed – Should Not Be Distributed |
| Zhuhai Livzon Diagnostics, Inc. | Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2) (Colloidal Gold) | Removed – Should Not Be Distributed* |
| Zybio Inc. | SARS-CoV-2 IgM/IgG Antibody Assay Kit (Colloidal Gold Method) | Removed – Should Not Be Distributed |

Site Notifications/Chat:
- Telegram Post Updates @JourneyToABetterLife (channel)
- Telegram Chatroom @JourneyBetterLifeCHAT (say hi / share info)
- Gettr Post Updates @chesaus (like fakebook)
Videos: