NZ Informed Consent document
I wonder how many doctors ignored the Informed Consent document during a certain jab rollout before recommending it to their patients: knowing the safety was unknown, that it was known to be experimental, with new technology, new methods of action, new adjuvants, new lipid nanoparticles, as well as secret IP-protected ingredients, that is still in clinical trial, and that it’s literally written into the contracts that the adverse events and long-term effects are completely unknowable?
I wonder if they knew that it wasn’t even shown to be effective even back when the tv was telling people it was over 90%? I wonder if they knew how many died or were seriously injured in the first 3 months of the trial before it was rolled out to the public? I wonder if they knew anything at all and warned any of their patients?
I wonder if the blame will shift to the doctors to take the fall when the truth comes to light?
How much did your profits increase by selling your soul? Was it worth it?
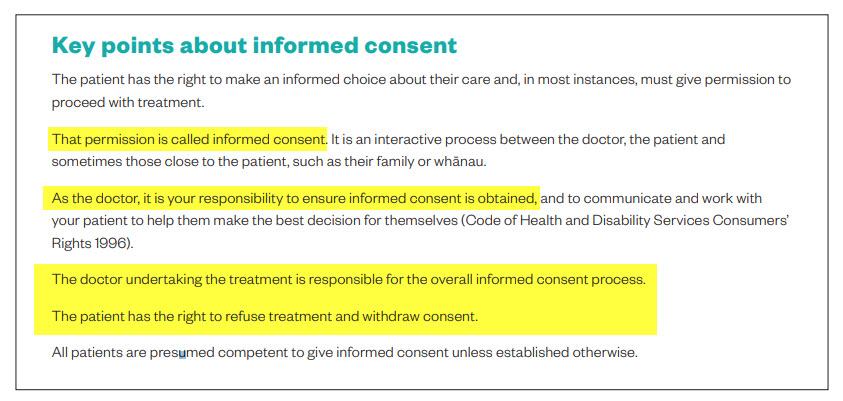
The doctor undertaking the treatment is responsible for the overall informed consent process
As the doctor, it is your responsibility to ensure informed consent is obtained, and to communicate and work with your patient to help them make the best decision for themselves (Code of Health and Disability Services Consumers’ Rights 1996).
In most situations, treatment should only go ahead if:
- your patient has received all the information that is relevant to their decision, and
- you are sure that your patient understands that information and the consequences of their decision.
Questions to consider before going ahead with treatment:
- What is your patient’s understanding of their condition and the outcome they are hoping to achieve?
- Have you (or another colleague) explained the different treatment options including the risks and benefits of each option, and the option of not treating (adopting a see what happens with time approach)?
- Have you given your patient relevant information that would influence how they would decide?
- If your patient is unsure about your advice or recommendations, are they aware that they can seek a second opinion?
- If a proposed treatment is new, experimental or lacks scientific evidence, have you explained this to your patient?
- Has your patient had enough time to ask questions and think about how they would like to proceed?
- Does your patient have additional needs (disability, language barriers, low health literacy) and need more support to make a decision?
Browse:

Site Notifications/Chat:
- Telegram Post Updates @JourneyToABetterLife (channel)
- Telegram Chatroom @JourneyBetterLifeCHAT (say hi / share info)
- Gettr Post Updates @chesaus (like fakebook)
Videos:
References