TGA, FDA, EMA, MHRA: drug regulators for hire (BMJ)
The six leading medical regulators, in Australia, Canada, Europe, Japan, the UK, and the US, are “funded by the very industries” they are meant to regulate. TGA in Australia gets 96% of their budget from the industry they are supposed to regulate.
The British Medical Journal has just published a feature investigational piece by Maryanne Demasi to expose the drug regulators. (01)
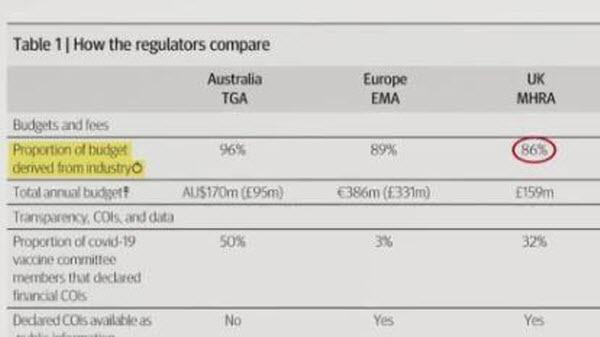
Of the six regulators, Australia had the highest proportion of budget from industry fees (96%) and in 2020-2021 approved more than nine of every 10 drug company applications.
Europe’s EMA derives 89% and UK’s MHRA derives 86%!
Australia’s Therapeutic Goods Administration (TGA) firmly denies that its almost exclusive reliance on pharmaceutical industry funding is a conflict of interest (COI). In response to a query, the agency said, “All fees and charges are prescribed in our legislation. To provide transparency, the TGA fees and charges are published on the TGA website.”

“The TGA should not be relying on the analysis of that data produced by the drug companies. Rather the TGA should be reanalysing the source data,” says Lexchin. “Further, the TGA should be holding public hearings before new drugs are approved so that it can hear from members of the public and outside scientists.” ( Facebook Post by Senator Gerard Rennick )
Over the past decades, regulatory agencies have seen large proportions of their budgets funded by the industry they are sworn to regulate.
In 1992, the US Congress passed the Prescription Drug User Fee Act (PDUFA), allowing industry to fund the US Food and Drug Administration (FDA) directly through “user fees” intended to support the cost of swiftly reviewing drug applications. With the act, the FDA moved from a fully taxpayer funded entity to one supplemented by industry money. Net PDUFA fees collected have increased 30 fold—from around $29m in 1993 to $884m in 2016. (02)
Sociologist Donald Light of Rowan University in New Jersey, US, who has spent decades studying drug regulation, says, “Like the FDA, the TGA was founded to be an independent institute. However, being largely funded by fees from the companies whose products it is charged to evaluate is a fundamental conflict of interest and a prime example of institutional corruption.” Light says the problem with drug regulators is widespread.
Wow TGA has an annual budget of 170 million dollars derived from industry — but still uses “the sponsor’s” data to approve … “the sponsor’s drug or vaccine”?
The TGA, for example, says it conducts its covid-19 vaccine assessments based on “the information provided by the vaccine’s sponsor. ” According to a FOI request from last May, the TGA said it had not seen the source data from the covid-19 vaccine trials.
“Assessments of patient data is not usually undertaken by the regulators. Instead they use summaries provided by the pharmaceutical companies.”

In Australia, the membership of the TGA’s Advisory Committee on Vaccines is published on the agency’s website. The forms for recording past and current financial and non-financial interests are not, however, made public. A Freedom of Information (FOI 2289) Act request for their financial disclosures in August 2020 had names and details of the disclosures redacted. (03) (04)
Concern over COIs is not just directed at those who work for the regulators but extends to the advisory panels intended to provide regulators with independent expert advice. A BMJ investigation last year found several expert advisers for covid-19 vaccine advisory committees in the UK and US had financial ties with vaccine manufacturers—ties the regulators judged as acceptable. A large study that investigated the impact of COIs among FDA advisory committee members over 15 years found that those with financial interests solely in the sponsoring firm were more likely to vote in favour of the sponsor’s product, and that people who served on advisory boards solely for the sponsor were significantly more likely to vote in favour of the sponsor’s product. (05) (06) (07)
At the FDA, generally regarded as the world’s premier regulator, nine out of 10 of its past commissioners between 2006 and 2019 went on to secure roles linked with pharmaceutical companies, and its 11th and most recent, Stephen Hahn, is working for Flagship Pioneering, a company that acts as an incubator for new biopharmaceutical companies. (08)
While historical drug disasters like sulfanilamide and thalidomide raised the stature of regulatory agencies, Light argues regulators now need their own watchdog and is calling for a drug and vaccine safety board, independent of the drug regulator, with the authority, staffing, and funds to investigate incidents of patient harm. “Countries have independent safety boards for airlines and their passengers. Why not for drugs and patients too?” says Light.

See also:
Last 10 Posts tagged “Conflict$ of Interest“:
- C19-Vax-Lies Chat (Epidemiologist & Toxicologist)
- Senator Roberts calls out Prof Macartney’s $65M Vax Conflict$
- Flashback: Feb 2020 – Thank you China for posting the genetic code…
- Pfizer switched templates that could cause permanent genome changes to those injected & their offspring…[Senate Hearing]
- The Great Awakening [Documentary]
- Define: Vanguard
- Aussie Banks owned by Vanguard & BlackRock
- Wind Farms Con
- Following the $CIENCE? (Bookmarks)
- Harris says the quiet part out loud…

Site Notifications/Chat:
- Telegram Post Updates @JourneyToABetterLife (channel)
- Telegram Chatroom @JourneyBetterLifeCHAT (say hi / share info)
- Gettr Post Updates @chesaus (like fakebook)
Videos:
References